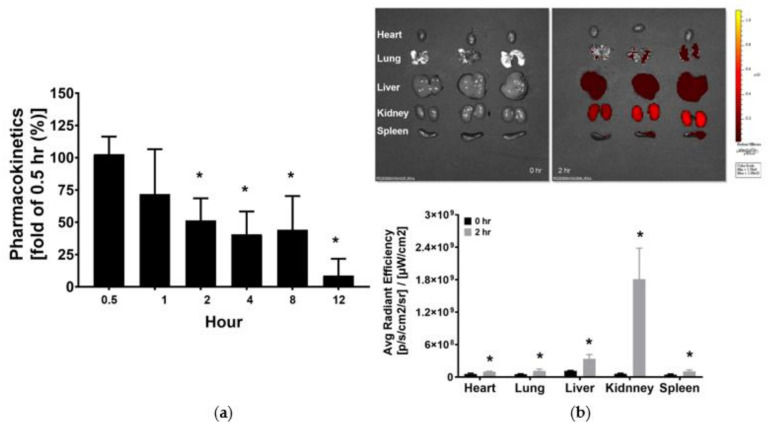
Figure 1.
(a) Pharmacokinetic analysis of KCF18. The plasma concentrations of KCF18 were measured through the assay of liquid chromatography–tandem mass spectrometry. The KCF concentration measured 0.5 h after intraperitoneal (i.p.) administration was used as the baseline. (b) Biodistribution of KCF18. The fluorescence signal intensities of KCF18 were measured 0 h (baseline) and 2 h after i.p. administration through the assay of the ex vivo bioluminescence imaging method. Data regarding pharmacokinetics and biodistribution were obtained from four and three mice from each time point, respectively. * p < 0.05 relative to the baseline.