Abstract
Simple Summary
The recent spreading of the invasive Asian hornet (Vespa velutina) to the Iberian Peninsula has led to the application of management measures to control and mitigate its impact on receiving environments. Among the most used control methods are capture traps, which use a sugary attractant to catch the invasive wasps. However, although the used V. velutina traps are presumably specific, they do not only attract V. velutina specimens, but also a large number of non-target species that are also captured. In the present work, the species of insects that unintentionally fall into the capture traps of V. velutina have been specifically identified, as well as their implications for ecosystem and for human activities. A total of 74 non-target taxa of insects were caught by the V. velutina trapping in northern Spain. Most of them were flies, mosquitoes, wasps and moths, being all highly important groups from the biological, ecological and economical points of view. Surprisingly, the most abundant trapped species was the invasive fly, Drosophila suzukii that represented the 36.07% of the total catches. Furthermore, we reported the first record of ectoparasitic mites of the genus Varroa on V. velutina, constituting a newly recorded symbiotic association.
Abstract
The introduction of invasive species is considered one of the major threats to the biodiversity conservation worldwide. In recent years, an Asian invasive species of wasp has set off alarms in Europe and elsewhere in the world, Vespa velutina. The Asian wasp was accidentally introduced in France around 2004 and shortly thereafter it was able to colonise practically all of Europe, including the Iberian Peninsula. The ecological and economic implications of V. velutina invasion and its high colonisation ability have triggered widespread trapping campaigns, usually supported by beekeepers and local governments, with the aim of diminishing its population and its negative impacts. Among the most used control methods are the capture traps, which use a sugary attractant to catch the invasive wasps. However, the species-specific selectivity and efficiency of these traps has been little studied. In this paper, we have analysed the specific identity of the unintentionally trapped insect species from northern Spain (covering one-year period), as well as we have assessed the provided ecosystem services by them. A total of 74 non-target taxa of insects were caught by the V. velutina studied traps, most of them correspond to the orders Diptera, Hymenoptera and Lepidoptera, the dipterans being the most abundant group. Surprisingly, the most abundant trapped species was the invasive fly, Drosophila suzukii that represented the 36.07% of the total catches. Furthermore, we reported the first record of ectoparasitic mites of the genus Varroa on V. velutina, constituting a newly recorded symbiotic association. Hopefully, the provided information helps to develop new protocols and management tools to control this invasive species in the Iberian Peninsula and other temperate areas of western Europe and the Mediterranean basin.
Keywords: invasive species, exotic species, non-target insects, services, disservices, Varroa, biodiversity, Iberian Peninsula, Spain, taxonomy
1. Introduction
Ongoing human activities, such as agricultural intensification and associated land use changes, habitat destruction and fragmentation, global warming and the spreading of invasive species are causing extensive shifts in native biodiversity worldwide [1,2,3,4,5,6,7,8,9,10]. Biological invasions constitute a multiple threat for biodiversity, economic activities, and even the human health. In recent years, an invasive species of wasp—Vespa velutina Lepeletier, 1836—has set off alarms in Europe and elsewhere in the world [1,2,3,4,5,6,7]. This famous species, commonly known as yellow-legged wasp or Asian wasp, is native to eastern Asia and belongs to the family Vespidae. Vespa velutina was accidentally introduced with ceramic boxes from China into Europe around 2004, where it was firstly detected in the French area of Lot-et-Garonne [1,2]. Since its successful introduction in France, it has rapidly colonised other European countries such as Spain, Portugal, Belgium, Italy, the United Kingdom, The Netherlands and Germany. Its rapid dispersal has been explained by the fact that V. velutina has not enough autochthonous direct competitors or predators; the local inexhaustible food sources; its high reproduction rates, and the European climate conditions that favour its proliferation and spread into new areas [3,4].
Vespa velutina was detected for the first time in Spain in 2010 in Amaiur (Navarra, northern Spain) and since then it was able to colonize practically the whole northern half of Spain, from Galicia to Catalonia [5,6]. The Asian wasp is considered as a threat to current biodiversity. It is a generalist predator of medium-sized insects like other Hymenoptera and Diptera, showing a special predilection for the European honeybee (Apis mellifera Linnaeus, 1758) [1,2]. After its rapid and successful invasion, the Asian wasp has exterminated entire beehives of the A. mellifera, decreasing thereby the availability and supply of apiculture products and causing substantial losses in this economic sector [7]. Also, V. velutina outcompetes with the autochthonous European hornet V. crabro Linnaeus, 1758 for the same ecological niche and food sources with consequent ecological repercussion [11,12]. On the other hand, like other vespids, their bite can cause serious health problems for allergy sufferers [12]. Its invasion has triggered important socio-economic impacts [7,13] and thus, the Asian wasp was officially considered as an invasive species and was included in the Spanish Catalogue of Invasive Species (Catálogo Español de Especies Invasoras, R D 630/2013). Furthermore, it is listed as an invasive alien species of Union concern (EU Regulation 1141/2016) in the framework of the respective European regulation (EU Regulation 1143/2014). After the arrival of the Asian wasp in Europe, serious concern has arisen as to the real impact on local insect populations due to its predatory nature [14], especially after the knowledge about its predilection to prey on European honeybees [1,2].
Consequently, government administrations of several European countries are monitoring the spreading dynamics of V. velutina, aiming to detect their early presence on newly colonised areas and to implement control strategies to prevent/reduce their expansion. Currently, the main control strategies are based on trapping adults, using different types of traps and baits, and nest detection and destruction [13,14,15,16,17]. Paradoxically, there are few studies on the efficiency and selectiveness of these traps and their attractants on the Asian hornet [18,19]. It is known (although not species-specifically quantified) that the currently utilized traps do not only attract V. velutina specimens, but also other species of insects, such as other hymenopterans (bees, wasps and allies) and members of the order Diptera (flies and mosquitoes) [1,2,13,18,19]. Furthermore, the proportion of these non-target insects in V. velutina traps stress the need of developing alternative monitoring and control techniques [18]. Dipteran and hymenopteran species are essential for the proper ecosystem functioning, as well as for being providers of great benefits to the agriculture (e.g., the production of entomophilous crops) and other human activities [18,19,20,21]. However, as a consequence of the little published/available information on this regard, the potential impacts of catches of certain groups of insects (e.g., the pollinators) cannot be properly assessed. Thus, it is crucial to know the specific identity of the entomofauna affected by the V. velutina traps, as well as the functional roles that they play in the ecosystem.
The main goal of this work is to assess the specific diversity of the non-target insect species captured by V. velutina traps, taking the Principado de Asturias (northern Spain) as a study case. This study aims as well to assess the functional roles of the trapped insects (ecosystem services and disservices) in the ecosystem and their status (native, exotic, invasive), in order to achieve a better understanding of the real effects of V. velutina traps in the ecosystem. Hopefully, this information helps to develop new protocols and management tools to control this invasive species in the Iberian Peninsula and other temperate areas of western Europe and the Mediterranean basin.
2. Materials and Methods
Diverse environmental factors (i.e., land cover, habitat structure, or human disturbance) can influence the effectiveness of the baited traps, increasing the chances to capture V. velutina in particular areas, such as rural localities where the governmental trapping campaigns were carried out in northern Spain (see https://www.asturias.es/general/-/categories/613173?p_r_p_categoryId=613173 accessed on 4 May 2021). Thus, for the study of the diversity of insects unintentionally captured by V. velutina traps, we analysed eight monitoring traps located in a plot with a majority plantation of apple trees located in a rural area of Loredo, Asturias, northern Spain (43°24′ N–6°44′ W, 310 m altitude) (Figure 1). At this locality, V. velutina (Figure 1e) has been present since 2016.
Figure 1.
Location of the sampling traps in Asturias (northern Spain) (a); General view of the plot (b); detail of “Vespa Catch” type trap placed on the branch of an apple tree in the study location (c); sample of captured insects in one of the studied traps (d); Vespa velutina specimen from the study site (e).
The trap type used was the VespaCatch® trap made by Véto-pharma (Palaiseau, France, Figure 1b,c) and the bait was VespaCatch® attractant made also by Véto-pharma (for additional information see https://www.blog-veto-pharma.com/ accessed on 4 May 2021). We selected this trap type and attractant because they were the ones used by the Principado de Asturias Regional Government during the trapping campaigns that they carry out annually (see https://www.asturias.es/general/-/categories/613173?p_r_p_categoryId=613173 accessed on 1 May 2021). Traps were positioned on apple trees at a height of 1.5 m from the ground (Figure 1b), with a distance of 10 m between them. The traps covered one-year period, from April 2020 to March 2021. They were activated during four consecutive weeks (30 trapping days each) in four seasons: spring, summer, autumn and winter. The traps were checked every 4–5 days, at the same time, the attractant was renewed, and the trapped fauna (Figure 1d) were collected and temporarily stored in 70% ethanol until pinned for taxonomic identification. All collected specimens were identified to species level except for some lepidopteran specimens that had a high degree of deterioration and for the members of the families Phoridae, Sciaridae and Formicidae, which require molecular studies for the proper specific determination. For the specific identification, both external and internal (e.g., genitalia) morphological characters were examined under both dissecting stereomicroscope and compound light microscope. Selected specimens were photographed with a DFC310FX camera (Leica, Wetzlar, Germany) mounted on a Leica M205FA stereomicroscope. The specimens were identified by the authors and subsequently deposited at the Zoological Collection of the Department of Organisms and System (BOS) of the University of Oviedo (https://bos.uniovi.es/artropodos accessed on 1 May 2021).
For the community analysis, species abundance data were analysed, using PRIMER v. 6 community analysis software. To visualize the differences in species composition among seasons a matrix of similarity was constructed by means of Bray Curtis similarity coefficient [22], as well as a cluster (group-average mode) and a multidimensional scaling (MDS). SIMPER analysis was carried out to identify the species that characterized the different groups. The phenological study was carried out by plotting the abundances of the most common and representative species of each season to check biological patterns.
3. Results
3.1. Specific Diversity of the Non-Target Insect Species
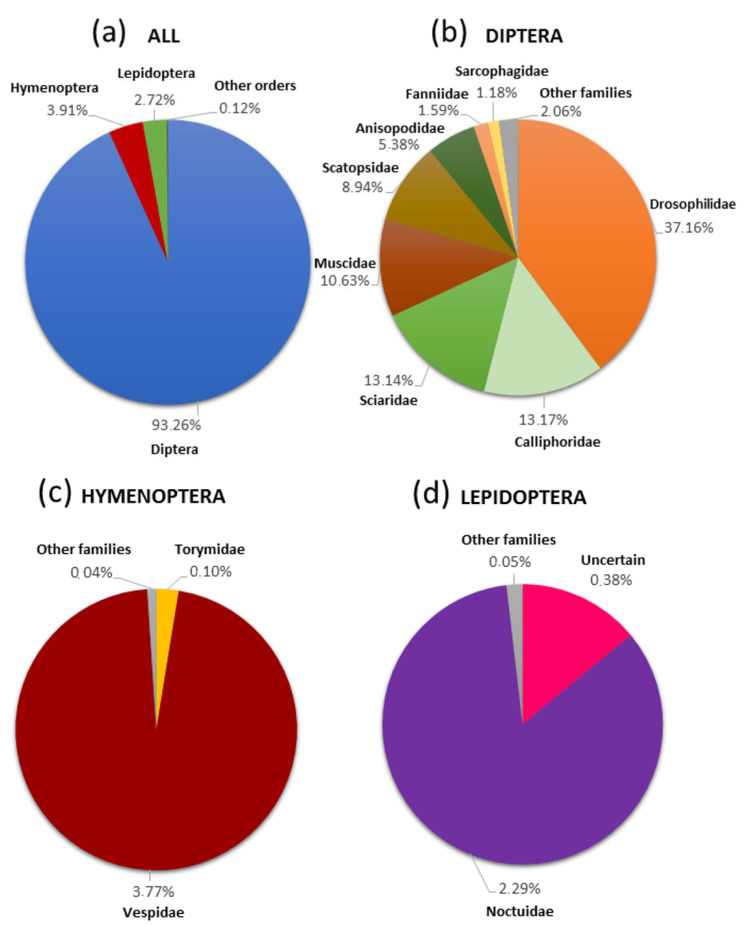
A total of 12,835 trapped specimens were collected and examined. They belonged to 74 different taxa grouped into seven arthropod orders (Table 1; Figure 2a). The order Diptera was the most abundant in the traps, representing 93.26% of the total samples and harbouring 53 taxa (Figure 1b). Within dipterans the most common species caught was the invasive species Drosophila suzukii (Matsumura, 1931), explaining the 36.07% of the total catches. The order Hymenoptera was the second group most affected by traps, constituting 3.91% of the total and affecting 10 species (Figure 1c). Among hymenopterans, the percentage of V. velutina caught was 2.23% and one of its congener V. crabro Linnaeus, 1758 was 1.45%. The incidence of other hymenopteran species was anecdotal, representing only 0.23% of the total sample (Table 1). The trapped species of the genera Vespula and Torymus had abundance percentages of 0.09% and 0.1% respectively. The order Lepidoptera was the third group most affected, constituting 2.72% of the total and involving at least 5 taxa (some lepidopteran specimens cannot be identified due to their high degree of deterioration) (Figure 1d). In relation to the butterflies caught, we can highlight the presence of two invasive species, the true armyworm moth (Mythimna unipuncta (Haworth, 1809)) and the geranium bronze (Cacyreus marshalli Butler, 1897), whose abundances in the total sample were 2.29% and 0.03% respectively.
Table 1.
Overall trapped species (total) and species per season. For each season, the number of collected specimens per trap and the percentage of trapped specimens per species total number of trapped insects are shown.
| Order | Family | Species | Author | N. of Specimens (Spring) | % of Insects (Spring) | N. of Specimens (Summer) | % of Insects (Summer) | N. of Specimens (Autumn) | % of Insects (Autumn) | N. of Specimens (Winter) | % of Insects (Winter) | N. of Specimens (Total) | % of Insects (Total) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diptera | Anisopodidae | Sylvicola cinctus | (Fabricius, 1787) | 32 | 0.25% | 70 | 0.55% | 34 | 0.26% | 71 | 0.55% | 207 | 1.61% |
| Diptera | Anisopodidae | Sylvicola fuscatus | (Fabricius, 1775) | 5 | 0.04% | 15 | 0.12% | 32 | 0.25% | 1 | 0.01% | 53 | 0.41% |
| Diptera | Anisopodidae | Sylvicola punctatus | (Fabricius, 1787) | 72 | 0.56% | 139 | 1.08% | 118 | 0.92% | 102 | 0.79% | 431 | 3.36% |
| Diptera | Calliphoridae | Bellardia viarum | (Robineau-Desvoidy, 1830) | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Diptera | Calliphoridae | Bellardia vulgaris | (Robineau-Desvoidy, 1830) | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Diptera | Calliphoridae | Calliphora vicina | Robineau-Desvoidy, 1830 | 39 | 0.30% | 122 | 0.95% | 31 | 0.24% | 123 | 0.96% | 315 | 2.45% |
| Diptera | Calliphoridae | Calliphora vomitoria | (Linnaeus, 1758) | 1 | 0.01% | 7 | 0.05% | 0 | 0.00% | 13 | 0.10% | 21 | 0.16% |
| Diptera | Calliphoridae | Chrysomya albiceps | (Wiedemann, 1819) | 2 | 0.02% | 3 | 0.02% | 0 | 0.00% | 2 | 0.02% | 7 | 0.05% |
| Diptera | Calliphoridae | Lucilia ampullacea | Villenueve, 1922 | 1 | 0.01% | 8 | 0.06% | 6 | 0.05% | 1 | 0.01% | 16 | 0.12% |
| Diptera | Calliphoridae | Lucilia caesar | (Linnaeus, 1758) | 189 | 1.47% | 488 | 3.80% | 63 | 0.49% | 183 | 1.43% | 923 | 7.19% |
| Diptera | Calliphoridae | Lucilia illustris | Meigen, 1826 | 4 | 0.03% | 8 | 0.06% | 3 | 0.02% | 11 | 0.09% | 26 | 0.20% |
| Diptera | Calliphoridae | Morinia doronici | (Scopoli, 1763) | 1 | 0.01% | 4 | 0.03% | 1 | 0.01% | 0 | 0.00% | 6 | 0.05% |
| Diptera | Calliphoridae | Onesia floralis | Robineau-Desvoidy, 1830 | 0 | 0.00% | 7 | 0.05% | 0 | 0.00% | 0 | 0.00% | 7 | 0.05% |
| Diptera | Calliphoridae | Pollenia griseotomentosa | (Jacentkovsky, 1944) | 6 | 0.05% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 6 | 0.05% |
| Diptera | Calliphoridae | Pollenia labialis | Robineau-Desvoidy, 1830 | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Diptera | Calliphoridae | Pollenia rudis | (Fabricius, 1794) | 49 | 0.38% | 148 | 1.15% | 33 | 0.26% | 86 | 0.67% | 316 | 2.46% |
| Diptera | Calliphoridae | Stomorhina lunata | (Fabricius, 1794) | 15 | 0.12% | 29 | 0.23% | 0 | 0.00% | 0 | 0.00% | 44 | 0.34% |
| Diptera | Chloropidae | Meromyza femorata | Macquart, 1835 | 0 | 0.00% | 3 | 0.02% | 0 | 0.00% | 0 | 0.00% | 3 | 0.02% |
| Diptera | Chloropidae | Thaumatomyia notata | (Meigen, 1830) | 5 | 0.04% | 3 | 0.02% | 9 | 0.07% | 24 | 0.19% | 41 | 0.32% |
| Diptera | Drosophilidae | Drosophila melanogaster | Meigen, 1830 | 9 | 0.07% | 20 | 0.16% | 22 | 0.17% | 84 | 0.65% | 135 | 1.05% |
| Diptera | Drosophilidae | Drosophila suzukii | (Matsumura, 1931) | 676 | 5.27% | 2629 | 20.48% | 744 | 5.80% | 580 | 4.52% | 4629 | 36.07% |
| Diptera | Drosophilidae | Gitona distigma | Meigen, 1830 | 0 | 0.00% | 3 | 0.02% | 0 | 0.00% | 2 | 0.02% | 5 | 0.04% |
| Diptera | Fanniidae | Fannia canicularis | (Linnaeus, 1761) | 1 | 0.01% | 10 | 0.08% | 42 | 0.33% | 36 | 0.28% | 89 | 0.69% |
| Diptera | Fanniidae | Fannia cf. postica | (Stein, 1895) | 25 | 0.19% | 4 | 0.03% | 36 | 0.28% | 50 | 0.39% | 115 | 0.90% |
| Diptera | Lauxanidae | Sapromyza opaca | Becker, 1895 | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% | 2 | 0.02% |
| Diptera | Muscidae | Dasyphora albofasciata | (Macquart in Webb & Berthelot, 1839) | 18 | 0.14% | 61 | 0.48% | 1 | 0.01% | 2 | 0.02% | 82 | 0.64% |
| Diptera | Muscidae | Mesembrina meridiana | (Linnaeus, 1758) | 2 | 0.02% | 1 | 0.01% | 1 | 0.01% | 1 | 0.01% | 5 | 0.04% |
| Diptera | Muscidae | Musca autumnalis | (De Geer, 1776) | 1 | 0.01% | 3 | 0.02% | 10 | 0.08% | 53 | 0.41% | 67 | 0.52% |
| Diptera | Muscidae | Musca domestica | Linnaeus, 1758 | 9 | 0.07% | 24 | 0.19% | 8 | 0.06% | 38 | 0.30% | 79 | 0.62% |
| Diptera | Muscidae | Musca tempestiva | Fallén, 1817 | 0 | 0.00% | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Diptera | Muscidae | Muscina levida | (Harris, 1780) | 17 | 0.13% | 44 | 0.34% | 32 | 0.25% | 37 | 0.29% | 130 | 1.01% |
| Diptera | Muscidae | Muscina pascuorum | (Meigen, 1826) | 6 | 0.05% | 22 | 0.17% | 1 | 0.01% | 0 | 0.00% | 29 | 0.23% |
| Diptera | Muscidae | Muscina prolapsa | (Harris, 1780) | 48 | 0.37% | 110 | 0.86% | 11 | 0.09% | 34 | 0.26% | 203 | 1.58% |
| Diptera | Muscidae | Muscina stabulans | (Fallén, 1817) | 18 | 0.14% | 31 | 0.24% | 0 | 0.00% | 1 | 0.01% | 50 | 0.39% |
| Diptera | Muscidae | Mydaea scutellaris | Robineau-Desvoidy, 1830 | 12 | 0.09% | 46 | 0.36% | 7 | 0.05% | 2 | 0.02% | 67 | 0.52% |
| Diptera | Muscidae | Mydaea urbana | (Meigen, 1826) | 2 | 0.02% | 3 | 0.02% | 3 | 0.02% | 0 | 0.00% | 8 | 0.06% |
| Diptera | Muscidae | Neomyia cornicina | (Fabricius, 1781) | 3 | 0.02% | 4 | 0.03% | 1 | 0.01% | 3 | 0.02% | 11 | 0.09% |
| Diptera | Muscidae | Phaonia bitincta | Róndani, 186 | 53 | 0.41% | 87 | 0.68% | 2 | 0.02% | 24 | 0.19% | 166 | 1.29% |
| Diptera | Muscidae | Phaonia pallida | (Fabricius, 1787) | 100 | 0.78% | 202 | 1.57% | 0 | 0.00% | 0 | 0.00% | 302 | 2.35% |
| Diptera | Muscidae | Polietes lardarius | Fabricius, 1781 | 3 | 0.02% | 0 | 0.00% | 8 | 0.06% | 152 | 1.18% | 163 | 1.27% |
| Diptera | Muscidae | Pyrellia vivida | Robineau-Desvoidy, 1830 | 0 | 0.00% | 0 | 0.00% | 2 | 0.02% | 0 | 0.00% | 2 | 0.02% |
| Diptera | Phoridae | Phoridae sp. | 6 | 0.05% | 27 | 0.21% | 22 | 0.17% | 59 | 0.46% | 114 | 0.89% | |
| Diptera | Sarcophagidae | Blaesoxipha cf. rossica | Villeneuve, 1912 | 1 | 0.01% | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 2 | 0.02% |
| Diptera | Sarcophagidae | Sarcophaga aff. argyrostoma | (Robineau–Desvoidy, 1830) | 6 | 0.05% | 10 | 0.08% | 0 | 0.00% | 2 | 0.02% | 18 | 0.14% |
| Diptera | Sarcophagidae | Sarcophaga cf. haemorrhoidalis | Böttcher, 1913 | 9 | 0.07% | 20 | 0.16% | 6 | 0.05% | 2 | 0.02% | 37 | 0.29% |
| Diptera | Sarcophagidae | Sarcophagidae sp. | 19 | 0.15% | 44 | 0.34% | 16 | 0.12% | 16 | 0.12% | 95 | 0.74% | |
| Diptera | Scathophagidae | Scathophaga stercolaria | (Linnaeus, 1758) | 3 | 0.02% | 0 | 0.00% | 11 | 0.09% | 61 | 0.48% | 75 | 0.58% |
| Diptera | Scatopsidae | Reichertella pulicaria | (Loew, 1846) | 141 | 1.10% | 229 | 1.78% | 115 | 0.90% | 663 | 5.17% | 1148 | 8.94% |
| Diptera | Sciaridae | Sciaridae sp. | 209 | 1.63% | 518 | 4.04% | 523 | 4.07% | 436 | 3.40% | 1686 | 13.14% | |
| Diptera | Sirphidae | Myatropa florea | (Linnaeus, 1758) | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% | 0 | 0.00% | 1 | 0.01% |
| Diptera | Tabanidae | Tabanus sudeticus | Zeller, 1842 | 0 | 0.00% | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Diptera | Trichoceridae | Trichocera annulata | Meigen, 1818 | 0 | 0.00% | 0 | 0.00% | 2 | 0.02% | 0 | 0.00% | 2 | 0.02% |
| Diptera | Ulidiidae | Physiphora alceae | (Preyssler, 1791) | 4 | 0.03% | 18 | 0.14% | 2 | 0.02% | 1 | 0.01% | 25 | 0.19% |
| Hymenoptera | Torymidae | Torymus sinensis | (Muller, 1764) | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 5 | 0.04% | 5 | 0.04% |
| Hymenoptera | Torymidae | Torymus auratus | Kamijo, 1892 | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 8 | 0.06% | 8 | 0.06% |
| Hymenoptera | Apidae | Apis mellifera | (Linnaeus, 1758) | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Hymenoptera | Apidae | Bombus terrestris lusitanicus | Krüger, 1956 | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Hymenoptera | Formicidae | Plagiolepsis sp. | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% | |
| Hymenoptera | Formicidae | Solenopsis sp. | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% | 0 | 0.00% | 1 | 0.01% | |
| Hymenoptera | Vespidae | Vespa crabro | Linnaeus, 1758 | 25 | 0.19% | 9 | 0.07% | 136 | 1.06% | 16 | 0.12% | 186 | 1.45% |
| Hymenoptera | Vespidae | Vespa velutina | Lepeletier, 1836 | 81 | 0.63% | 6 | 0.05% | 112 | 0.87% | 87 | 0.68% | 286 | 2.23% |
| Hymenoptera | Vespidae | Vespula germanica | (Fabricius, 1793) | 2 | 0.02% | 0 | 0.00% | 5 | 0.04% | 0 | 0.00% | 7 | 0.05% |
| Hymenoptera | Vespidae | Vespula vulgaris | (Linnaeus, 1758) | 3 | 0.02% | 0 | 0.00% | 2 | 0.02% | 0 | 0.00% | 5 | 0.04% |
| Lepidoptera | Uncertain | Lepidoptera sp. 1 | 17 | 0.13% | 31 | 0.24% | 0 | 0.00% | 0 | 0.00% | 48 | 0.37% | |
| Lepidoptera | Uncertain | Lepidoptera sp. 2 | 0 | 0.00% | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% | |
| Lepidoptera | Licenidae | Cacyreus marshalli | Butler, 1897 | 0 | 0.00% | 1 | 0.01% | 3 | 0.02% | 0 | 0.00% | 4 | 0.03% |
| Lepidoptera | Noctuidae | Mythimna unipuncta | (Haworth, 1809) | 62 | 0.48% | 92 | 0.72% | 94 | 0.73% | 46 | 0.36% | 294 | 2.29% |
| Lepidoptera | Nymphalidae | Pararge aegeria | (Linnaeus, 1758) | 2 | 0.02% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 2 | 0.02% |
| Blattodea | Blattidae | Blatta orientalis | Linnaeus, 1758 | 0 | 0.00% | 0 | 0.00% | 4 | 0.03% | 0 | 0.00% | 4 | 0.03% |
| Hemiptera | Pentatomidae | Nezara viridula | Linnaeus, 1758 | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Mecoptera | Panorpidae | Panorpa communis | Linnaeus, 1758 | 0 | 0.00% | 2 | 0.02% | 1 | 0.01% | 0 | 0.00% | 3 | 0.02% |
| Neuroptera | Chrysopidae | Pseudomellada clathratus | (Schneider, 1845) | 0 | 0.00% | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 1 | 0.01% |
| Neuroptera | Chrysopidae | Pseudomellada flavifrons | (Brauer, 1851) | 1 | 0.01% | 4 | 0.03% | 0 | 0.00% | 0 | 0.00% | 5 | 0.04% |
| Neuroptera | Chrysopidae | Pseudomellada marianus | (Navás, 1905) | 1 | 0.01% | 1 | 0.01% | 0 | 0.00% | 0 | 0.00% | 2 | 0.02% |
| 2024 | 15.77% | 5375 | 41.88% | 2317 | 18.05% | 3114 | 24.26% | 12,835 | 100% |
Figure 2.
Percentages of the different orders of insects trapped in this study (a) and families most representative of the orders Diptera (b), Hymenoptera (c) and Lepidoptera (d).
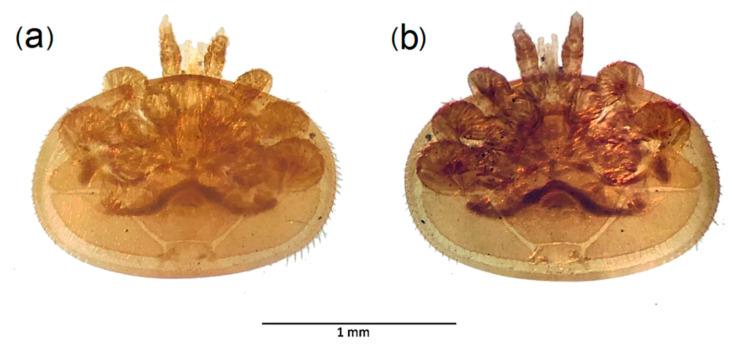
In addition to the mentioned insect species, three species of other taxonomic groups were found, i.e., Pomatias elegans (Müller, 1774) (Mollusca: Gastropoda: Pomatiidae), Dicranopalpus ramosus (Simon, 1909) (Arthropoda: Chelicerata: Arachnida: Opiliones) and a spider of the Family Salticidae (Arthropoda: Chelicerata: Arachnida: Araneae). Ectoparasitic mites of the genus Varroa (Arachnida: Acari: Parasitiformes) were found attached on five specimens of the 286 examined V. velutina specimens (one mite per V. velutina specimen). The prevalence rate of the Varroa parasite in V. velutina was of 0.017 (1.75%). In all cases, the mites were found clinging to the lateral-ventral part of the abdomen of V. velutina specimens. Additionally, two free specimens of the same mite genus were found in the attractant liquid from the traps. Varroa specimens found measured between 1.5 and 2 mm and corresponded with two colour morphotypes, one with darker colour (reddish-brown) and the other lighter (amber color) (Figure 3). The analysis of their morphological features showed that they were consistent with the diagnosis of the genus Varroa. This finding constitutes the first record of this genus of parasitic mites on V. velutina, presenting a newly recorded symbiotic association.
Figure 3.
Micrographs of ethanol preserved specimens of Varroa found on Vespa velutina. Dorsal view of Varroa(a) and ventral view of same (b).
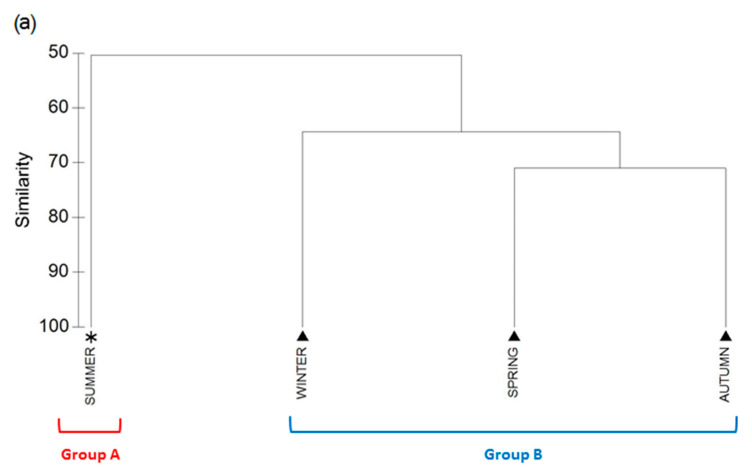
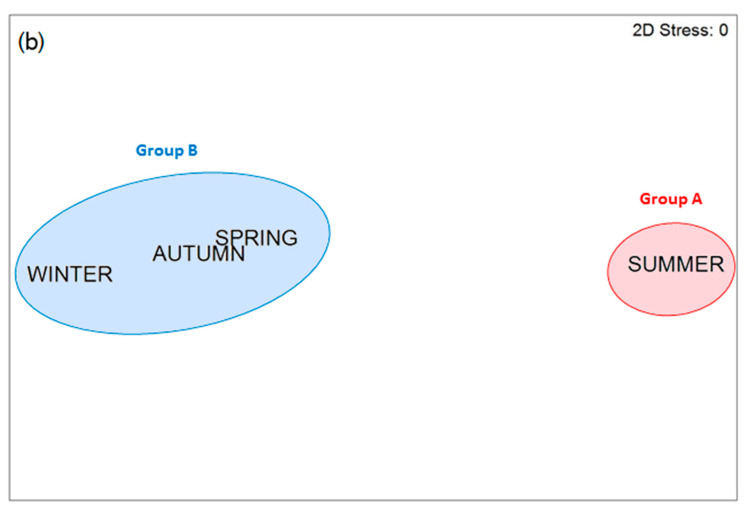
Cluster analysis carried out with abundance data showed two assemblages, Group A (Summer) and Group B (spring, autumn and winter) (Figure 4). Group A clustered the samples of summer and included 53 taxa.
Figure 4.
Cluster dendrogram (a) and MDS plot (b) based on the similarity of the insect’s abundance among seasons. Symbols identify the different assemblages as defined by branches in the dendrogram. Ellipses encapsulate all samples from the same assemblage in the MDS plot.
Group B gathered the samples from spring, autumn and winter, with an average similarity of 66.5%. The taxa richness was 67, being D. suzukii (Diptera) and Family Sciaridae (Diptera) the ones that mostly contribute with 37.59% and 16.93% respectively. The SIMPER analysis showed a 49.67% of dissimilarity between Group A and Group B. This is accounted for by the major representativeness of D. suzukii and Lucilia caesar (Diptera) with percentages of 50.35% and 8.83% respectively (Table A1).
3.2. Ecosystem Services and Disservices
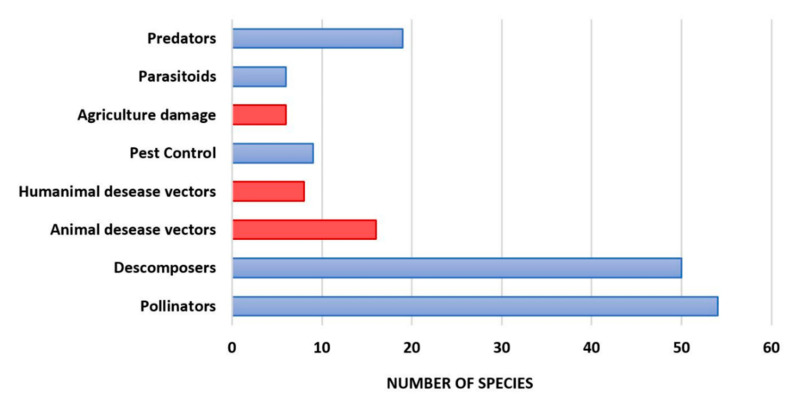
The results obtained from the analysis of services and disservices showed that 73% and 67.6% of the trapped species were pollinators and decomposers, respectively. Predatory species (25.7%) were also compromised to a great extent. The proportion of species that produce disservices was quite high (Figure 5).
Figure 5.
Number of species that perform the different ecosystem services (blue) and disservices (red) among those found in the studied sample.
Among them, we can highlight those that cause damage to agriculture (8.1%) and those that act as vectors of both animal (21.6%) and human (10.8%) diseases (Figure 5).
3.3. Phenological Study
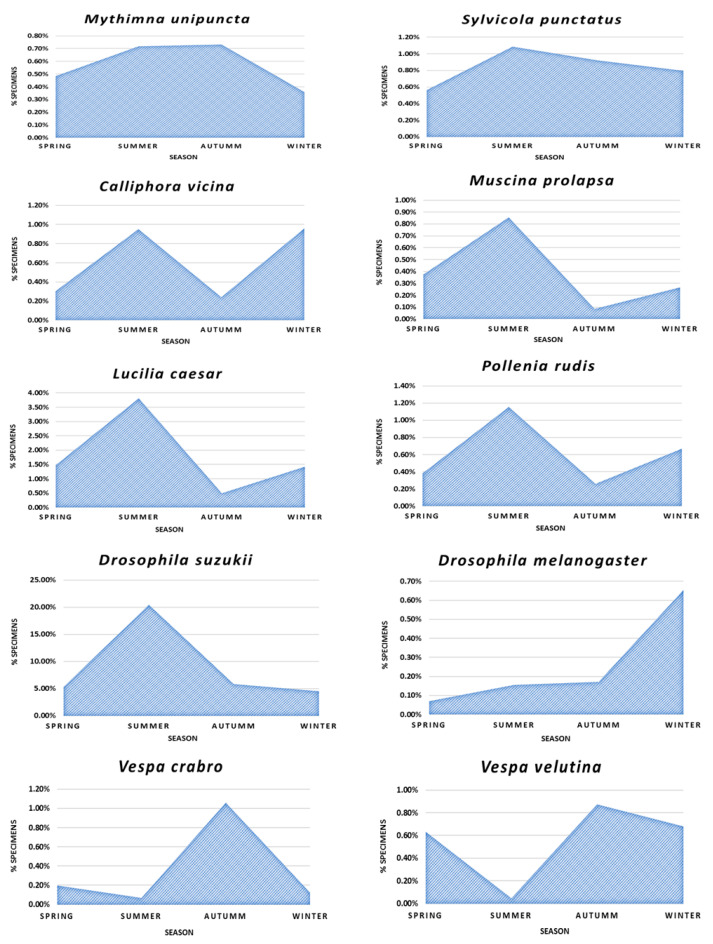
For the phenological analysis (Figure 6), the most abundant and representative species of the total sample were selected, i.e., seven species of Diptera (L. caesar, Pollenia rudis, Calliphora vicina, Muscina prolapsa, D. suzukii, D. melanogaster and Sylvicola punctatus), the lepidopteran Mythimna unipuncta and the hymenopterans V. velutina and V. crabro.
Figure 6.
Phenological analysis of the most abundant and representative non-target species of the total sample during the four seasons of the year based on the percentage of the total number of individuals.
The presence of both species of the genus Vespa (V. velutina and V. crabro) were confirmed throughout the whole year, showing similar phenological cycles and an abundance maximum in autumn. Vespa velutina was also a slightly lower in spring (Figure 6). Queens of both species were only found during the spring season, the remaining specimen captures corresponded to workers and to a lesser extent to males.
The phenological analyses showed that M. unipuncta reach the maximun number of individuals in summer and autumn and the rest of the year remains in intermediate abundances. The species L. caesar, P. rudis, C. vicina and M. prolapsa, present very similar life cycles, reaching the maximum number of individuals in summer and falling drastically in autumn, apart from S. punctatus, whose number of individuals decreases in a more attenuated way, reaching its minimum in winter. However, C. vicina, seems to present a biannual cycle, presenting two maxima, one in summer and the other in spring. Drosophila suzukii present its maximum during the summer and reach very low abundances in autumn and winter. However, D. melanogaster, shows an inverse trend reaching its maximum in winter.
4. Discussion
The results of this study have shown that V. velutina traps, apart from its primary function (i.e., to control wasp populations), can be also used for detection of new invasions of insect pests and/or parasites, for delimitation of infestation areas and for monitoring population levels of established pests of non-target species. This information can be used to make decisions on the initiation of control measures or to measure effectiveness of a pest management program. In this study, the careful analysis of the traps has shown the occurrence of a new symbiotic relationship between V. velutina and Varroa mites. Varroa jacobsoni Oudemans, 1904 is a parasite that feeds on the hemolymph of bees of the genus Apis [23]. It was first described in Indonesia parasitising the Asian honeybee (Apis cerana Fabricius, 1793) [23,24], although it can also parasitise other hymenopterans such as wasps of the genus Vespula (Jeliński, 1990). In 2000, Anderson and Trueman [23] conducted a molecular study that showed that V. jacobsoni was actually a species complex and a new species was described, V. destructor Anderson & Trueman, 2000. Currently it is considered that V. destructor is mainly responsible for the damage to the beekeeping sector, since it is commonly found on European honeybees (A. mellifera) [23]. Furthermore, V. destructor acts as a vector for the wing strain virus (DWV) that can lead to the death of an entire colony of bees or hymenopterans [23]. Its role as a vector for another RNA virus, the Moku virus (MV), that affects wasps of the genus Vespula has recently been discovered [25]. Both viruses, DWS and MV, are generalists and may infect a wide variety of insects [25]. Thus, the relationship between V. velutina and Varroa should be studied further, since the Asian wasp could constitute a new dispersal vector of Varroa and its potential carried viruses.
On the other hand, our results have shown that the insect orders most affected by V. velutina trapping in northern Spain are Diptera, Hymenoptera, and Lepidoptera, confirming previous studies [18,19]. These three orders are very important groups in terms of the provided ecosystem services (Figure 5; Table 2). It is noteworthy that six dipteran families are of special interest from an economic point of view due to their agricultural, veterinary, or medical involvement [26,27]. Also, agronomically they are of great interest as important pollinating group, and some species are crop-pests or help to control them [26,27,28]. In relation to the hymenopterans, it is well-known that they play fundamental roles, outstanding is their role in the pollination process, their great agricultural implication, and their use as parasitoids for the pest control [29]. The order Lepidoptera is another large group involved in pollination and some butterfly families are of special interest due to their agricultural involvement, since the larvae of most species are phytophagous and thus being commonly pests in crops and in forest stands [27,28].
Table 2.
List of species found with the ecological service/disservice provided and their 2018status’ (exotic and invasive species are highlighted in red colour). PO: pollinator; DE: decomposer; ADV: animal disease vector; HDV: human disease vector; PC: pest control; AD: agriculture damage; PA: parasitoids; PR: predators; NA: not applicable.
| Scientific Name | PO | DE | ADV | HDV | PC | AD | PA | PR | Status |
|---|---|---|---|---|---|---|---|---|---|
| Sylvicola cinctus | + | Native | |||||||
| Sylvicola fuscatus | + | Native | |||||||
| Sylvicola punctatus | + | Native | |||||||
| Bellardia viarum | + | + | Native | ||||||
| Bellardia vulgaris | + | + | Native | ||||||
| Calliphora vicina | + | + | + | Native | |||||
| Calliphora vomitoria | + | + | + | Native | |||||
| Chrysomya albiceps | + | + | + | Native | |||||
| Lucilia ampullacea | + | + | Native | ||||||
| Lucilia caesar | + | + | + | Native | |||||
| Lucilia illustris | + | + | + | Native | |||||
| Morinia doronici | + | + | Native | ||||||
| Onesia floralis | + | + | Native | ||||||
| Pollenia griseotomentosa | + | + | Native | ||||||
| Pollenia labialis | + | + | Native | ||||||
| Pollenia rudis | + | + | Native | ||||||
| Stomorhina lunata | + | + | + | Native | |||||
| Meromyza femorata | + | Native | |||||||
| Thaumatomyia notata | + | + | + | + | Native | ||||
| Drosophila melanogaster | + | Native | |||||||
| Drosophila suzukii | + | + | Invasive | ||||||
| Gitona distigma | + | Native | |||||||
| Fannia canicularis | + | Native | |||||||
| Fannia postica | + | Native | |||||||
| Sapromyza opaca | + | + | Native | ||||||
| Dasyphora albofasciata | + | + | Native | ||||||
| Mesembrina meridiana | + | + | + | Native | |||||
| Musca autumnalis | + | + | + | + | Native | ||||
| Musca domestica | + | + | + | + | Native | ||||
| Musca tempestiva | + | + | + | + | Native | ||||
| Muscina levida | + | + | + | + | + | Native | |||
| Muscina pascuorum | + | + | + | + | + | Native | |||
| Muscina prolapsa | + | + | + | + | + | Native | |||
| Muscina stabulans | + | + | + | + | + | Native | |||
| Mydaea Scutellaris | + | + | + | Native | |||||
| Mydaea urbana | + | + | + | Native | |||||
| Neomyia cornicina | + | + | Native | ||||||
| Phaonia bitincta | + | + | Native | ||||||
| Phaonia pallida | + | + | Native | ||||||
| Polietes lardarius | + | + | Native | ||||||
| Pyrellia vivida | + | + | Native | ||||||
| Phoridae sp.1 | + | NA | |||||||
| Blaesoxipha cf. rossica | + | + | Native | ||||||
| Sarcophaga aff. argyrostoma | + | + | Native | ||||||
| Sarcophaga cf. haemorrhoidalis | + | + | Native | ||||||
| Sarcophagidae sp. | + | + | NA | ||||||
| Scathophaga stercolaria | + | + | + | + | Native | ||||
| Reichertella pulicaria | + | Native | |||||||
| Sciaridae sp. | + | + | NA | ||||||
| Myatropa florea | + | + | Native | ||||||
| Tabanus sudeticus | + | + | + | Native | |||||
| Trichocera annulata | Native | ||||||||
| Physiphora alceae | + | Native | |||||||
| Torymus sinensis | + | + | Exotic | ||||||
| Torymus auratus | + | + | Native | ||||||
| Apis mellifera | + | Native | |||||||
| Bombus terrestris lusitanicus | + | Native | |||||||
| Plagiolepsis sp. | Native | ||||||||
| Solenopsis sp. | Native | ||||||||
| Vespa crabro | + | + | Native | ||||||
| Vespa velutina | + | + | Invasive | ||||||
| Vespula germanica | + | + | Native | ||||||
| Vespula vulgaris | + | + | Native | ||||||
| Lepidoptera sp. 1 | + | NA | |||||||
| Lepidoptera sp. 2 | + | NA | |||||||
| Cacyreus marshalli | + | + | Invasive | ||||||
| Mythimna unipuncta | + | + | Invasive | ||||||
| Pararge aegeria | + | Native | |||||||
| Blatta orientalis | + | + | Native | ||||||
| Nezara viridula | + | Native | |||||||
| Panorpa communis | + | + | Native | ||||||
| Pseudomallada clathratus | + | + | + | Native | |||||
| Pseudomallada flavifrons | + | + | + | Native | |||||
| Pseudomallada marianus | + | + | + | Native |
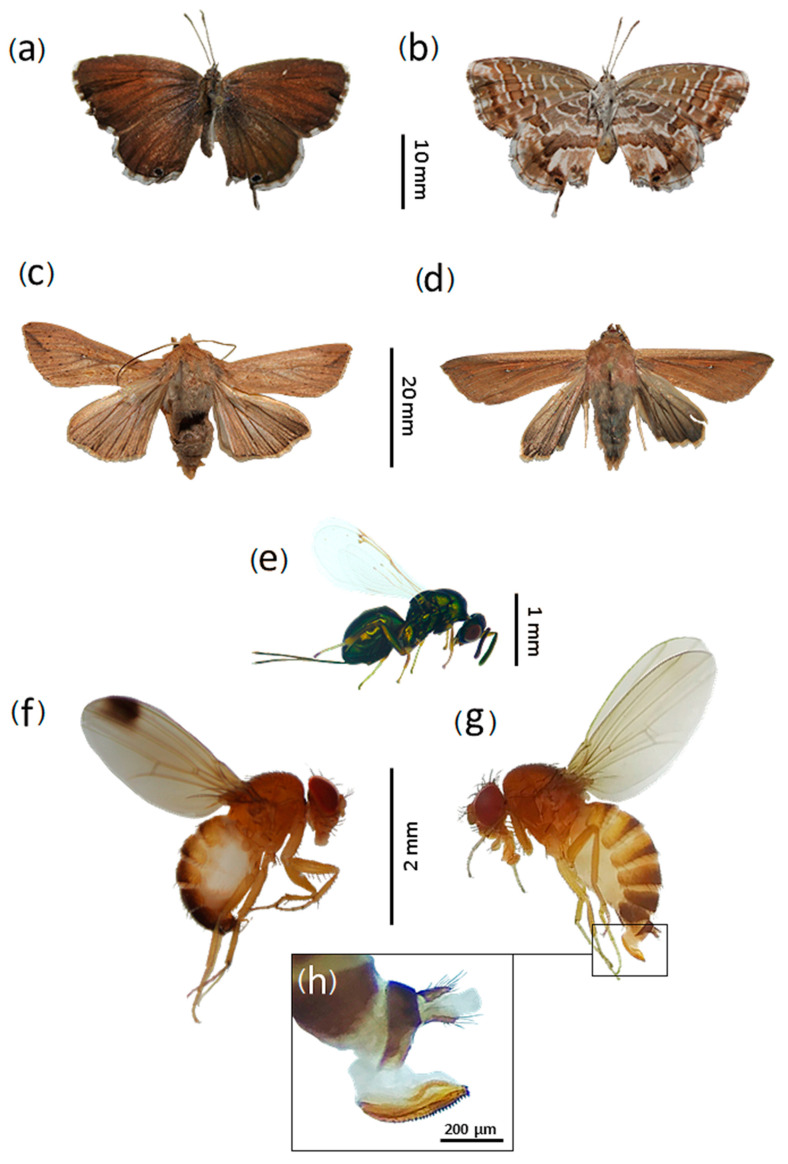
Here, we have reported a specific richness of non-target species of insects in V. velutina traps of 74 taxa. Previous studies of non-target insects affected by V. velutina traps show similar trends to those reported here, the most affected groups being the orders Diptera, Hymenoptera and Lepidoptera [18,19]. However, in both studies a large number of ant specimens (Family Formicidae) were found, even exceeding the total number of dipterans [18], while in this work only two specimens were trapped. Among the identified species, four were exotic or non-indigenous to Spain, C. marshalli (Figure 7a,b), M. unipuncta (Figure 7c,d), D. suzukii (Figure 7f–h), and T. sinensis (Figure 7e), being the first three, in addition, invasive. Drosophila suzukii was the most captured species in studied V. velutina traps, with 4629 specimens caught and representing the 36.1% of the total catches (Table 1). Drosophila suzukii is an indigenous species from Asia (China, Japan, Korea, and Thailand) [30]. After its introduction to Spain in 2008, it caused serious damage to thin-skinned fruits such as cherries, blueberries, raspberries, blackberries and strawberries, both in cultured growing areas and in natural environments [30,31]. These damages are produced by its oviscapt morphology (Figure 7c), since it presents a series of denticles that allow D. suzukii to attack healthy fruits and not only those that are ripe or in decomposition process, as is usual in native Drosophila species [31,32]. This is especially relevant since D. suzukii may cause the ‘human intestinal myiasis’, due to the ingestion of apparently healthy fruits infected with larvae of this species [31,32]. The introduction of this species in Europe is related to the importation of fruit from East Asian countries [31]. The high abundance of this species during the summer may be related to the appearance of the fleshy fruits that it attacks. It is worth noting that D. melanogaster, its native congener, showed a completely inverse trend to D. suzukii, showing a low general abundance through the year and reaching its maximum in winter (Figure 6). Cacyreus marshalli, commonly known as geranium bronze, is a native species of Africa (Botswana, South Africa, Mozambique, Zimbabwe, and Lesotho) [33]. In its native range it frequents garden areas where they feed on Geraniaceae, a family of plants that includes genera such as Geranium and Pelargonium [34]. The first record of this species in Europe dated back to 1989 from the Balearic Islands, where it was reported as a plague of the ornamental geraniums (Pelargonium) [35]. However, shortly thereafter, it has been shown that, in the absence of the ornamental Pelargonium species, C. marshalli can attack native species of the genus Geranium, and furthermore they can outcompete with indigenous lycaenid species, such as Aricia nicias Meigen, 1830 and Eumedonia eumedon Esper, 1780 [34]. The presence of this species in the studied samples was very low, only four specimens were trapped (0.03% of the total). Finally, the true armyworm moth, M. unipuncta, it is an autochthonous species of North America (USA and Canada) [36]. This moth has great agricultural importance and is considered a pest species due to their damage in corn, oat, rice and other grass crops (caused by its larvae, known as soldier worms) [36,37].
Figure 7.
Exotic and invasive species captured in the studied V. velutina traps. Cacyreus marshalli (Lepidoptera: Lycaenidae) dorsal view (a); ventral view of the same (b); Male of Mythimna unipuncta (Lepidoptera: Noctuidae) (c); female of the same (d); female of Torymus sinensis (Hymenoptera: Torymidae) (e); male of Drosophila suzukii (Diptera: Drosophilidae) (f); female of the same (g); detail of the oviscapt of female D. suzukii (h).
5. Conclusions
The present work has constituted a pioneering study on the species-specific diversity of non-target species of the traps used to control the invasive V. velutina in northern Spain. These findings may be extended/compared to other parts of the Iberian Peninsula and to temperate regions of southern Europe (both with similar entomofauna). Specific entomofaunal identification studies, although laborious, are very informative and essential to mitigate the ecological repercussions of the control activities V. velutina may generate. Since, as it is well-known most of the non-target species play important ecological roles as pollinators and decomposers, among others. If we only considered the provided services/disservices by the non-target insect species found, V. velutina traps look very detrimental since they catch many species that play important ecological roles. However, on a broader view, most of the species are caught in very-low numbers, and if we look at the numeric contribution of individuals, the invasive species become dominant. The orders of insects most affected by the trapping of V. velutina were Diptera, Hymenoptera and Lepidoptera, being the invasive species D. suzukii, the most abundant species in the traps and representing 36.07% of the total catches. Concerning hymenopterans, ‘vespa-catch’ (veto-pharma) attractant showed to be selective for wasps of the genus Vespa (and Vespula to a lesser extent) since the capture of other hymenopteran species was practically anecdotal. Although the most affected hymenopteran by traps was the target species V. velutina (accounted for 2.23% of catches), the European species V. crabro was the following species, the second hornet species in the account, with an abundance in traps of 1.45%. As for the lepidoptera, the presence of the exotic species, M. unipuncta, which was found during the whole annual cycle, stands out due to its large size and by the fact that when these moths fall into the trap, they immediately swell and greatly increase their volume, and subsequently saturate the traps (reducing its effectiveness). Finally, the newly reported relationship between V. velutina and the ectoparasite mites of the genus Varroa needs to be studied further, since the Asian wasp could constitute a new dispersal vector of this parasite and its potential carried viruses.
Acknowledgments
We specially thank Piluca Álvarez and Jairo Robla for providing key references and for their advice with identification of some insect species, Jesús Arias for help in the field sampling and Hannelore Paxton for the English editing of the manuscript. We are also grateful to two anonymous reviewers for helpful comments and suggestions.
Appendix A
Table A1.
SIMPER analysis of Group A (summer) and Group B (spring, autumn and winter). The dominant 12 insect species contributing to the similarity/dissimilarity of each group are tabulated.
| Cluster/Taxa | % Contribution |
|---|---|
| Group A similarity (66.55%) | |
| Drosophila suzukii | 37.59 |
| Sciaridae sp. | 16.93 |
| Reichertella pulicaria | 7.52 |
| Lucilia caesar | 6.18 |
| Vespa velutina | 5.05 |
| Sylvicola punctatus | 4.94 |
| Mythimna unipuncta | 3.17 |
| Pollenia rudis | 2.32 |
| Calliphora vicina | 2.05 |
| Sylvicola cinctus | 1.99 |
| Fannia cf. postica | 1.73 |
| Muscina levida | 1.31 |
| Group B (Less than 2 samples in group) | |
| Group A & B dissimilarity (66.55%) | |
| Drosophila suzukii | 50.35 |
| Lucilia caesar | 8.83 |
| Reichertella pulicaria | 5.22 |
| Phaonia pallida | 4.28 |
| Sciaridae sp. | 3.49 |
| Pollenia rudis | 2.39 |
| Vespa velutina | 2.24 |
| Muscina prolapsa | 2.03 |
| Calliphora vicina | 1.55 |
| Phaonia bitincta | 1.55 |
| Dasyphora albofasciata | 1.38 |
| Vespa crabro | 1.31 |
Author Contributions
Conceptualization, A.A.; methodology, A.A. and O.S.; formal analysis, A.A. and O.S.; investigation, A.A. and O.S.; resources, A.A. and O.S.; writing—original draft preparation, A.A. and O.S.; writing—review and editing, A.A. and O.S.; supervision, A.A.; project administration, A.A.; funding acquisition, A.A. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the project FUO-20-278 (Fundación Universidad de Oviedo—Dirección General de Medio Natural y Planificación Rural (Consejería de Medio Rural y Cohesión Territorial del Gobierno del Principado de Asturias)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haxaire J., Bouguet J.P., Tamisier J.P. Vespa velutina Lepeletier, 1836, une redoutable nouveauté pour la faune de France (Hym., Vespidae) Bull. Soc. Entomol. Fr. 2006;111:194. [Google Scholar]
- 2.Villemant C., Haxaire J., Streito J.C. The discovery of the Asian hornet Vespa velutina in France. (La découverte du frelon asiatique Vespa velutina, en France) Insectes. 2006;143:3–7. [Google Scholar]
- 3.Caragata C.R., Montesinos J.L.V. Datos ambientales preliminares del avispón asiático (Vespa velutina Lepeletier, 1836) (Hymenoptera, Vespidae) en Asturias, España. Bol. R. Soc. Esp. Hist. Nat. 2020;114:19–35. [Google Scholar]
- 4.Barbet-Massin M., Rome Q., Muller F., Perrard A., Villemant C., Jiguet F. Climate change increases the risk of invasion by the yellow-legged hornet. Biol. Conserv. 2013;157:4–10. doi: 10.1016/j.biocon.2012.09.015. [DOI] [Google Scholar]
- 5.Castro L., Pagola-Carte S. Vespa velutina Lepeletier, 1836 (Hymenoptera: Vespidae), recolectada en la Península Ibérica. Heteropterus Rev. Entomol. 2010;10:193–196. [Google Scholar]
- 6.Rodríguez-Flores M.S., Seijo-Rodríguez A., Escuredo O., Seijo-Coello M.C. Spreading of Vespa velutina in northwestern Spain: Influence of elevation and meteorological factors and effect of bait trapping on target and non-target living organisms. J. Pest. Sci. 2019;92:557–565. doi: 10.1007/s10340-018-1042-5. [DOI] [Google Scholar]
- 7.Monceau K., Bonnard O., Thiéry D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2014;87:1–16. doi: 10.1007/s10340-013-0537-3. [DOI] [Google Scholar]
- 8.Young J., Watt A., Nowicki P., Alard D., Clitherow J., Henle K., Johnson R., Laczko E., McCracken D., Matouch S., et al. Towards sustainable land use: Identifying and managing the conflicts between human activities and biodiversity conservation in Europe. Biodivers. Conserv. 2005;14:1641–1661. doi: 10.1007/s10531-004-0536-z. [DOI] [Google Scholar]
- 9.Henle K., Alard D., Clitherow J., Cobb P., Firbank L., Kull T., McCracken D., Moritz R.F.A., Niemelä J., Rebane M., et al. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe—A review. Agric. Ecosyst. Environ. 2008;124:60–71. doi: 10.1016/j.agee.2007.09.005. [DOI] [Google Scholar]
- 10.Monceau K., Maher N., Bonnard O., Thiéry D. Evaluation of competition between a native and an invasive hornet species: Do seasonal phenologies overlap? Bull. Entomol. Res. 2015;105:462–469. doi: 10.1017/S0007485315000280. [DOI] [PubMed] [Google Scholar]
- 11.López S., González M., Goldarazena A. Vespa velutina lepeletier, 1836 (Hymenoptera: Vespidae): First records in Iberian Peninsula. Bull. OEPP. 2011;41:439–441. doi: 10.1111/j.1365-2338.2011.02513.x. [DOI] [Google Scholar]
- 12.Haro L., Labadie M., Chanseau P., Cabot C., Blanc-Brisset I., Penouil F. Medical consequences of the Asian black hornet (Vespa velutina) invasion in Southwestern France. Toxicon. 2010;55:650–652. doi: 10.1016/j.toxicon.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Arca M., Papachristoforou A., Mougel F., Rortais A., Monceau K., Bonnard O., Tardy P., Thiéry D., Silvain J.F., Arnold G. Defensive behaviour of Apis mellifera against Vespa velutina in France: Testing whether European honeybees can develop an effective collective defense against a new predator. Behav. Process. 2014;106:122–129. doi: 10.1016/j.beproc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Budge G.E., Hodgetts J., Jones E.P., Ostojá-Starzewski J.C., Hall J., Tomkies V., Semmence N., Brown M., Wakefield M., Stainton K. The invasion, provenance and diversity of Vespa velutina Lepeletier (Hymenoptera: Vespidae) in Great Britain. PLoS ONE. 2017;12:e0185172. doi: 10.1371/journal.pone.0185172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leza M., Miranda M.Á., Colomar V. First detection of Vespa velutina nigrithorax (Hymenoptera: Vespidae) in the Balearic Islands (Western Mediterranean): A challenging study case. Biol. Invasions. 2018;20:1643–1649. doi: 10.1007/s10530-017-1658-z. [DOI] [Google Scholar]
- 16.Lioy S., Manino A., Porporato M., Laurino D., Romano A., Capello M., Bertolino S. Establishing surveillance areas for tackling the invasion of Vespa velutina in outbreaks and over the border of its expanding range. NeoBiota. 2019;46:51–69. doi: 10.3897/neobiota.46.33099. [DOI] [Google Scholar]
- 17.Laurino D., Lioy S., Carisio L., Manino A., Porporato M. Vespa velutina: An Alien Driver of HoneyBee Colony Losses. Diversity. 2020;12:5. doi: 10.3390/d12010005. [DOI] [Google Scholar]
- 18.Lioy S., Laurino D., Capello M., Romano A., Manino A., Porporato M. Effectiveness and Selectiveness of Traps and Baits for Catching the Invasive Hornet Vespa velutina. Insects. 2020;11:706. doi: 10.3390/insects11100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas-Nossa S.V., Novoa N., Serrano A., Calviño-Cancela M. Performance of baited traps used as control tools for the invasive hornet Vespa velutina and their impact on non-target insects. Apidologie. 2018;49:872–885. doi: 10.1007/s13592-018-0612-0. [DOI] [Google Scholar]
- 20.Primack R.B., Silander J.A. Measuring the relative importance of different pollinators to plants. Nature. 1975;255:143–144. doi: 10.1038/255143a0. [DOI] [Google Scholar]
- 21.Klein A.M., Vaissiere B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C., Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray J.R., Curtis J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957;27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 23.Anderson D.L., Trueman J.W.H. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000;24:165–189. doi: 10.1023/A:1006456720416. [DOI] [PubMed] [Google Scholar]
- 24.Jelinski M. Roztocz Varroa jacobsoni Oudemans, 1904 na larwach osy pospolitej Vespa (Paravespula) vulgaris L. Wiad Parazytol. 1990;36:55–58. [PubMed] [Google Scholar]
- 25.Mordecai G.J., Brettell L.E., Pachori P., Villalobos E.M., Martin S.J., Jones I.M., Schroeder D.C. Moku virus; a new Iflavirus found in wasps, honeybees and Varroa. Sci. Rep. 2016;6:34983. doi: 10.1038/srep34983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gállego B.J. Manual de Parasitología: Morfología y Biología de Los Parásitos de Interés Sanitario. Universidad de Barcelona; Barcelona, Spain: 2006. pp. 1–517. [Google Scholar]
- 27.Balachowsky A.S. In: Entomologie Appliquée à 1’Agriculture. Tome II. Lepidópteros, Deuxième Volume. Masson e.C., editor. Directeur De Publication; París, France: 1972. pp. 1345–1354. [Google Scholar]
- 28.Scoble M.J. The Lepidoptera. Form, Function and Diversity. Oxford University Press; Oxford, UK: 1992. pp. 1–404. [Google Scholar]
- 29.Southwick E.E., Southwick L. Estimating the economic value of honeybees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J. Econ. Entomol. 1992;85:621–633. doi: 10.1093/jee/85.3.621. [DOI] [Google Scholar]
- 30.Calabria G., Máca J., Bächil G., Serra L., Pascual M. First records of the potencial pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012;136:139–147. doi: 10.1111/j.1439-0418.2010.01583.x. [DOI] [Google Scholar]
- 31.Bieńkowski A.O., Orlova-Bienkowskaja M.J. Invasive Agricultural Pest Drosophila suzukii (Diptera, Drosophilidae) Appeared in the Russian Caucasus. Insects. 2020;11:826. doi: 10.3390/insects11110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiel R.A., Narganes A.G., Argüelles M.B. Incidencia de “Drosophila suzukii” en cultivos de arándano y frambuesa en Asturias. Phytoma España. 2014;258:49–53. [Google Scholar]
- 33.Paradiso F., Martelli F., Cerrato C., Ghidotti S., Ramona V., Canterino S., Ferracini C., Bonelli S. From Africa to the Alps: Risk assessment on an invasion by Cacyreus marshalli (Butler, 1898) J. Insect. Conserv. 2019;23:279–288. doi: 10.1007/s10841-019-00138-w. [DOI] [Google Scholar]
- 34.Quacchia A., Ferracini C., Bonelli S., Balletto E., Alma A. Can the Geranium Bronze, Cacyreus marshalli, become a threat for European biodiversity? Biodivers. Conserv. 2008;17:1429–1437. doi: 10.1007/s10531-008-9350-3. [DOI] [Google Scholar]
- 35.Eitschberger U., Stamer P. Cacyreus marshalli Butler, 1898, Eine neue Tagfalterart für sie Europaïsche Fauna. Lepidoptera, Lycaenidae) Atalanta. 1990;21:101–108. [Google Scholar]
- 36.Brou V.A., Brou C.D. Mythimna unipuncta (Haworth, 1809) (Lepidoptera: Noctuidae) in Louisiana. South. Lepid. News. 2020;42:31–33. [Google Scholar]
- 37.Hill D.S. Agricultural Insect Pest of the Tropics and Their Control. Alden Press; London, UK: 1983. pp. 1–749. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.