Abstract
Glomerular mesangial cell (MC) proliferation is one of the causative factors of glomerular diseases and one of their prominent pathological features. Rapamycin can inhibit MC proliferation and slow the progression to chronic renal fibrosis. The present study was designed to observe the role of rapamycin in MC proliferation and to explore the mechanism by which rapamycin acts on Akt and MAPK/ERK1/2 pathways in mesangial cells. MTT assay and flow cytometry were used to evaluate the proliferation and the cell cycle phase of glomerular mesangial cells respectively. The mRNA expression level of p70S6K was detected by RT-qPCR. Western blotting was performed to determine p70S6K, PI3K/Akt, and PI3K/MAPK protein expression. We found that rapamycin could reduce mesangial cell proliferation and arrest the cell cycle in the G1 phase, however the inhibition effect of 1000 nmol/L rapamycin was not higher than that in the 100 nmol/L group. The results of western blotting showed that 1000 nmol/L rapamycin more significantly inhibited the phosphorylation of p70S6K than 100 nmol/L, suggesting there should be another signaling pathway that activates the proliferation of MCs. Moreover, our results revealed that 1000 nmol/L rapamycin led to Raf1-MEK1/2-ERK pathway activation through a p70S6K-PI3K-mediated feedback loop in MCs. This study demonstrated that high-dose rapamycin leads to ERK1/2 activation through a p70S6K/PI3K/MAPK feedback loop in rat MCs, thus reducing the inhibitory effect of rapamycin on MC proliferation.
Keywords: rapamycin, mesangial cell, p70S6K/PI3K/MAPK, feedback loop
Introduction
Glomerular mesangial cells (MCs) play a crucial role in the glomerular capillary ultrafiltration apparatus and function in maintaining its structure.1 MCs change during the development of human glomerular diseases. Many glomerular diseases feature mesangial expansion and/or proliferation followed by glomerulosclerosis. Therefore, MC proliferation is considered an important part of the progression of glomerular diseases.2
Multiple signaling pathways function as regulators of MC proliferation, including the MAPK/ERK1/23 and PI3K/Akt/mTOR pathways.4 The PI3K/Akt/mTOR pathway regulates cell metabolism, growth, proliferation, migration, and survival.5 Upon the activation of mTORC1, its downstream effectors such as eukaryotic initiation factor-4E-binding protein-1 and p70 ribosomal S6 kinase (p70S6K) evoke certain responses. Through the interaction with these factors, mTORC1 controls cellular proliferation and leads to the promotion of DNA transcription, RNA translation, ribosomal biogenesis, and cell cycle progression.6
Rapamycin, implicated as an mTORC1 inhibitor, inhibits the proliferation of several cell types including lymphocytes, vascular smooth muscle cells, and endothelial cells.7 Our preliminary results showed that rapamycin could inhibit mesangial cell proliferation and ameliorate renal fibrosis and hypofunction in a model of rat IgA nephropathy.8 However, many clinical and experimental studies have shown that rapamycin and its analogs activate Akt and ERK1/2. For example, Filippone et al.9 determined that reperfusion therapy with rapamycin attenuates myocardial infarction through activating Akt and ERK. In addition, Chen et al.10 demonstrated that rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Furthermore, Soares et al.11 reported that there are different patterns of Akt and ERK feedback activation in response to rapamycin active site mTOR inhibitors and metformin in pancreatic cancer cells.
However, whether rapamycin can regulate Akt and ERK proteins to influence MC proliferation and the mechanisms behind this modulation have not been extensively studied. The present study was designed to observe the role of rapamycin in MC proliferation and to explore the mechanism by which rapamycin acts on Akt and MAPK/ERK1/2 pathways in mesangial cells.
Methods
Cell culture and treatments
TheHBZY-1 (rat mesangial cell line) was obtained from the China Center for Typical Collection (Wuhan, China). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 2 mM L-glutamine, 15 mM HEPES buffer, 10% fetal bovine serum, and penicillin (100 U/mL)/streptomycin (100 μg/mL) at 37°C with 5% CO2. The cells were synchronized by exposure to DMEM supplemented with 0.5% fetal bovine serum for 24 h. PDGF-BB at a concentration of 20 ng/mL was used to stimulate MC proliferation. At the same time, the cells were treated with varying concentrations (1–1000 nmol/L) of rapamycin (Adamas-beta, Switzerland). All reagents used for cell culture were purchased from Sigma (USA), unless otherwise noted.
Cell proliferation assay
The MTT assay was employed to assess the ability of cells to proliferate. MCs were cultured in 96-well plates (5 × 103 cells/well) for 24 h, and then co-stimulated with PDGF-BB (20 ng/mL) and rapamycin (1–1000 nmol/L) for 20 or 44 h. Then, 20 µl of MTT (5 mg/mL; Invitrogen, USA) was added to each well, followed by incubation for 4 h. After this, 150 µL of dimethyl sulfoxide (DMSO; Sigma) was added to each well to dissolve the formazan crystals. The optical density (OD) was measured at 492 nm. The inhibition rate was determined as follows: (1−ODdrug treatment/ODcontrol)×100%.
Flow cytometry
Flow cytometry was performed to evaluate the cell cycle and apoptosis. Cells were collected and digested into a cell suspension after 24 h of treatment, and then fixed with 70% ethanol at 4°C overnight. After three washes with PBS, they were treated with RNase (0.5 mg/mL) for 30 min at 37°C. Propidium iodide (PI, 50 µg/mL) was added to the cells and allowed to stain for 30 min at 4°C in the dark. The fluorescent signal of the cells was captured and analyzed using an FC500 flow cytometer (Beckman Coulter, Palo Alto, CA, USA) to determine the proportions of cells in the G1, S, and G2/M phases. For the apoptosis assay, cells were harvested after 24 h of treatment, and then prepared in suspension with 0.25% trypsin-EDTA. An Annexin V-FITC Apoptosis Detection Kit (Jiancheng, Nanjing, China) was used. The cells were incubated with Annexin V-FITC and PI for 15 min at room temperature in the dark. The apoptosis of the samples was also analyzed using an FC500 flow cytometer.
Real-time quantitative PCR
Cells were lysed in Trizol reagent (Invitrogen, USA) to isolate total RNA, following the manufacturer’s instructions, and then qualified using the Eppendorf BioPhotometer (Eppendorf, Germany). Before converting into cDNA, all samples underwent an additional step to remove genomic DNA. Next, 1 μg of RNA of each sample was reverse-transcribed into cDNA using cDNA Reverse Transcriptase (Takara, Japan). Gene expression was measured by RT-qPCR using RT reagent kit (Takara, Japan) with the respective primers. GADPH was employed as the housekeeping gene. The results were analyzed by the 2−ΔΔCt method and expressed as fold change in expression. The primer sequences used were as follows: GAPDH: forward TGAACGGGAAGCTCACTGG, reverse TCCACCACCCTGTTGCTGTA (targeted region: 134 bp); and p70S6K: forward ATGCTGCTTCTCGTCT, reverse TTGAGTCATCTGGGCTGT (targeted region: 204 bp).
Western blot analysis
The expression of the target protein was visualized by western blotting. Cells were collected 2 h after drug intervention and total protein was extracted using cell lysis buffer (KeyGEN, Nanjing, China). A portion (50 μg) of each sample was loaded on 10% differential SDS-polyacrylamide gels and separated by the application of a continuous voltage. After electrophoresis, the protein was transferred to a PVDF membrane according to the molecular weight. Then, each membrane was blocked with 5% skim milk. The membranes were next incubated with different primary antibodies (Cell Signaling Technology, USA; dilution 1:1000): p70 S6 kinase and phospho-p70 S6 kinase (Thr389); p44/42 MAPK (Erk1/2) (137F5) and phospho-p44/42MAPK (Erk1/2) (Thr202/Tyr204) (197G2); Akt antibody, phosphor-Akt (Thr308) and phosphor-Akt (Ser473); c-Raf antibody and phospho-c-Raf (Ser338) (56A6); and β-actin at 4°C overnight. Corresponding horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, USA) was incubated with the membranes for 2 h at room temperature. The densities of bands were quantified using the Quantity One analysis system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard error. For comparisons of multiple treatment groups, one-way ANOVA with Bonferroni correction was utilized. P < 0.05 was considered statistically significant.
Results
Rapamycin reduced mesangial cell proliferation
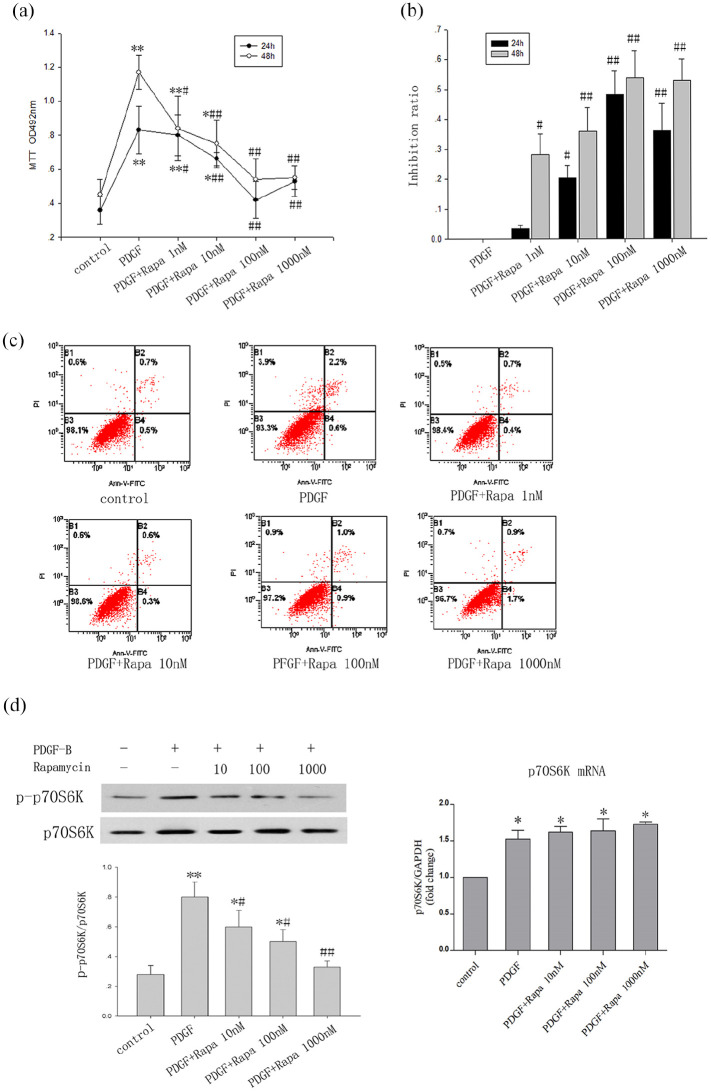
The MTT assay results indicated that PDGF-BB could induce MC proliferation, and rapamycin significantly inhibited this in both dose- and time-dependent manners. However, the time dependence gradually weakened with increasing dose (1–100 nmol/L). As shown in Figure 1b, the lowest rate at which rapamycin at 1 nmol/L inhibited MC proliferation was 28.2% at 48 h, but not 3.6% at 24 h (P < 0.01). In the 1000 nmol/L rapamycin group, the inhibition rates were not higher than those in the 100 nmol/L group, at either 24 or 48 h (24 h: 39.1% vs 48.1%; 48 h: 52.9% vs 53.8%) (Figure 1b). To identify the cause of the decrease in cell proliferation, we used flow cytometry to detect apoptosis. The results revealed that rapamycin had no obvious effect on apoptosis and there was no abundance of dead cells at 1000 nmol/L (Figure 1c).
Figure 1.
Rapamycin reduced mesangial cell proliferation and inhibited p70S6K phosphorylation. (a) MTT assay for cell proliferation; the optical density (OD) was measured at 492 nm. (b) The rate of inhibition of MC proliferation by rapamycin. Rapamycin inhibited cell proliferation induced by PDGF-BB in dose- and time-dependent manners at 24 and 48 h. Rapamycin treatment at 1 nmol/L for 48 h but not for 24 h significantly suppressed MC proliferation. There was no significant difference between the groups using 100 or 1000 nmol/L (n = 6/group). (c) Flow cytometry for cell apoptosis. Rapamycin had no obvious effect on apoptosis of MCs and there was no abundance of dead cells at 1000 nmol/L group. (d) Rapamycin (10–1000 nmol/L) significantly inhibited p70S6K phosphorylation by blocking the phosphorylation of mTOR in a dose-dependent manner . However, the test results for p70S6k mRNA showed that rapamycin does not affect its expression (Figure 1d)’.
The data are presented as the mean ± SD.
*P < 0.05. **P < 0.01 versus control.
#P < 0.05. ##P < 0.01 versus PDGF.
Effect of rapamycin on p-p70S6K
p70S6K, known as a downstream target of Akt/mTORC1, is frequently used to indicate the activity of mTORC1. The experimental results showed that PDGF-BB can upregulate the phosphorylation of p70S6K through the mTORC1 pathway. Compared with the findings in the group treated solely with PDGF-BB, rapamycin (10–1000 nmol/L) significantly inhibited p70S6K phosphorylation by blocking the phosphorylation of mTOR in a dose-dependent manner (Figure 1d). However, the test results for p70S6k mRNA showed that rapamycin does not affect its expression (Figure 1d).
Rapamycin arrested the cell cycle in the G1 phase
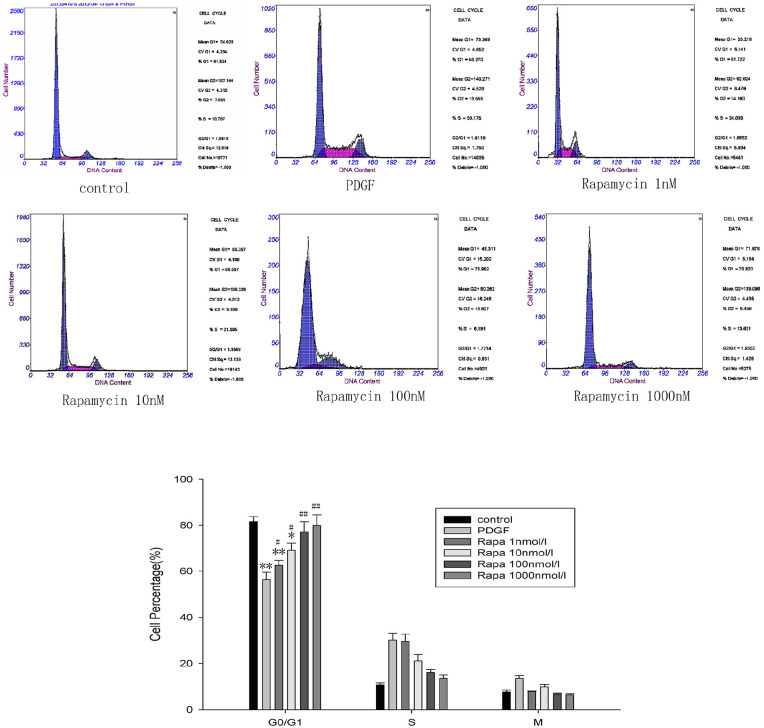
Cell cycle development plays an important role in the proliferation of MCs, but how rapamycin affects this progression is unclear. By applying flow cytometry, we revealed that PDGF-BB induced a change in the proportions of cells at the G1 and S phases. Specifically, the number of cells at the G1 phase decreased while those at the S phase increased, demonstrating that PDGF-BB promotes cell cycle progression. Rapamycin, by contrast, can reverse this phenomenon dose-dependently (1–100 nmol/L), blocking the transition of G1 cells to S phase (P < 0.05). As a result, the number of cells at S phase significantly decreased (P < 0.05). The transition of cells from one phase of the cell cycle to the next is important for cell proliferation, but rapamycin can block this by arresting cells at the G1 phase. However, when we increased the dose to 1000 nmol/L, the proportions of cells at different stages of the cell cycle did not differ significantly from those in the 100 nmol/L group (Figure 2).
Figure 2.
Rapamycin arrested the cell cycle in the G1 phase. Flow cytometry was used to analyze the cell cycle. Rapamycin dose-dependently (1–100 nmol/L) arrested cells in the G1 phase; therefore, the number of cells in the S phase was significantly lower (n = 6/group). However, in the 1000 nmol/L rapamycin group, the proportions of cells in different stages of the cell cycle were not significantly different from those in the 100 nmol/L group.
The data are presented as the mean ± SD.
*P < 0.05. **P < 0.01 versus control.
#P < 0.05. ##P < 0.01 versus PDGF.
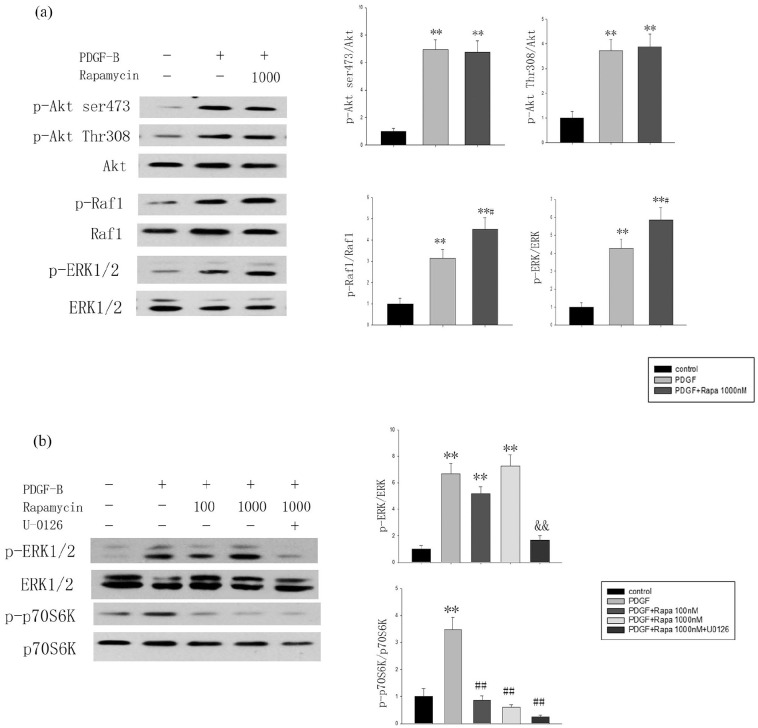
High-dose rapamycin activates Raf1-MEK-ERK but not PI3K/Akt(Thr308) and mTORC2-Akt(Ser473) in vitro
Substantial clinical and experimental evidence has shown that rapamycin and its analogs activate the negative feedback loop of PI3K/Akt(Thr308), mTORC2/Akt(Ser473), and PI3K/MAPK to attenuate the effects of rapamycin. Therefore, western blotting was performed to detect the phosphorylation of Akt(Thr308), Akt(Ser473), Raf1, and ERK1/2. Notably, PDGF-BB activated multiple signaling pathways to stimulate cell proliferation; the expression of p-Akt(Thr308), p-Akt(Ser473), p-Raf1, and p-ERK1/2 increased in the PDGF group compared with the levels in the control group (P < 0.05). After the intervention with 1000 nmol/L rapamycin, the phosphorylation of Raf1 and ERK1/2 increased more than that in the PDGF group (P < 0.05), but there were no significant differences in Akt(Thr308) or Akt(Ser473) phosphorylation between the PDGF- and rapamycin-treated groups (Figure 3a).
Figure 3.
High-dose rapamycin activated Raf1-MEK-ERK in MCs. (a) Akt(Thr308), Akt(Ser473), Raf1, and ERK1/2 phosphorylation status in starved (24 h, 0.1% FBS) MCs treated with vehicle or rapamycin (1000 nmol/L, 2 h) and stimulated with PDGF-BB (20 ng/mL, 2 h; n = 3). (b) Effect of rapamycin treatment (100 and 1000 nmol/L, 2 h) and/or UO126 (10 µmol/L, 2 h) on ERK and p70S6K phosphorylation in MCs (n = 3).
The data are presented as the mean ± SD.
**P < 0.01 versus control.
#P < 0.05. ##P < 0.01 versus PDGF.
&&P < 0.01 versus PDGF+rapamycin at 1000 nmol/L.
Next, the effect of rapamycin on ERK1/2 was further evaluated. Figure 3b shows that 100 nmol/L rapamycin had no effect on the expression of phosphorylated p44/42 ERK compared with that in the PDGF group, but p-ERK increased in the 1000 nmol/L group. The p-ERK1/2 that was induced by 1000 nmol/L rapamycin could be blocked by the MEK1/2 inhibition of UO126 (Figure 3b).
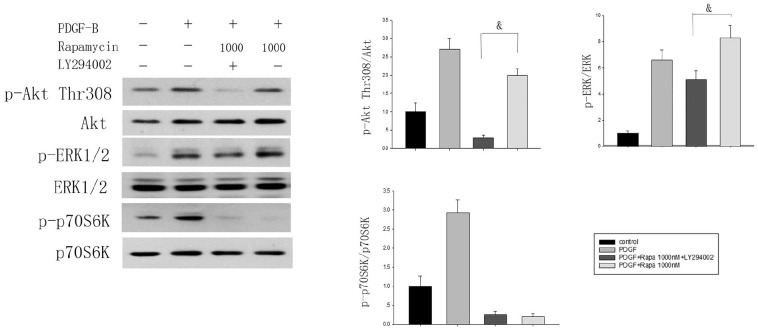
High-dose rapamycin-induced ERK1/2 activation is downstream of p70S6K/PI3K
The constitutively inactive form of p70S6K led to increased rapamycin-mediated ERK1/2 activation, indicating that p70S6K participates in the process of ERK1/2 activation by rapamycin. In line with this notion, we found that co-stimulation with 1000 nmol/L rapamycin and PDGF-BB could trigger the hyper-activation of ERK1/2, and pretreatment with an irreversible inhibitor of PI3K (10 µM LY294002) induced a reduction of ERK1/2 activation mediated by rapamycin. This suggested that PI3K participated in rapamycin-dependent ERK1/2 activation (Figure 4).
Figure 4.
Rapamycin-induced ERK1/2 activation occurs downstream of p70S6K/PI3K. The effects of rapamycin (1000 nmol/L, 2 h) and/or PI3K inhibitor LY294002 (10 µmol/L, 2 h) on Akt(Thr308), ERK, and p70S6K phosphorylation in MCs (n = 3) were analyzed by western blotting.
&P < 0.05.
Discussion
The mammalian target of rapamycin (mTOR), a defined downstream kinase of the PI3K/Akt pathway, plays an important role in regulating cell proliferation, growth, differentiation, migration, and survival.5,6 It has been reported that mTOR can be activated in several rat models of chronic kidney disease, such as compensatory renal hypertrophy,12 diabetic nephropathy,13 polycystic kidney disease,14 membranous nephropathy,15 anti-GBM glomerulonephritis,16 and anti-thy1 glomerulonephritis.17
Previous studies in our laboratory found that, in IgA nephropathy, the AKT/mTORC1/p70S6K pathway is activated, suggesting that rapamycin represents a potential treatment option.18 In IgA nephropathy, aggressive mesangial cell proliferation is an identified hallmark. Uncontrolled growth of mesangial cells appears to be a causative factor in progressive renal failure.19 Therefore, we cultured mesangial cells to explore the effect of rapamycin on their proliferation. We found that PDGF-BB-induced MC proliferation can be significantly suppressed by rapamycin in dose- (1–100 nmol/L) and time-dependent manners. In the 1000 nmol/L rapamycin-treated cells, the rate of inhibition of MC proliferation showed no significant difference compared with that in the 100 nmol/L group. Cell cycle analysis also showed the same results, but the western blotting results were different; 1000 nmol/L rapamycin more significantly inhibited the phosphorylation of p70S6K than 100 nmol/L did. At the same time, 1000 nmol/L rapamycin did not cause apoptosis or death. Therefore, in the group with 1000 nmol/L rapamycin, there may be another signaling pathway activating the proliferation of mesangial cells.
In cancer cells, it is well documented that mTORC1 inhibitors can activate a negative feedback loop.20–22 Through this loop, p70S6K initiates growth factor receptor, so the inhibition of mTORC1 results in enhanced activation of PI3K/AKT and the PI3K/MAPK pathways.23,24 This activation by the inhibition of mTORC1 in cancer cells led us to hypothesize that this feedback could also be a cause of the weakened antiproliferative activity of 1000 nmol/L rapamycin.
To determine the nature of the activation of PI3K/Akt and PI3K/MAPK by 1000 nmol/L rapamycin in MCs, we confirmed the existence of this signaling circuit. p70S6K activation causes degradation of the insulin receptor substrate (IRS) and subsequently downregulates PI3K signaling, while rapamycin can inhibit mTORC1, the factor initiating this negative feedback loop, thereby inhibiting this feedback loop and increasing PI3K signaling. Similarly, in another type of mTORC1-mediated signaling, owing to the blocking effect of rapamycin, increased phosphorylation of p70S6K and Rictor causes inhibition of mTORC2 activity and eventually activates Akt.25 At the same time, the inhibition of mTORC1 leads to MAPK pathway activation. Therefore, we tested the phosphorylation of these pathway proteins. Remarkably, the treatment with 1000 nmol/L rapamycin resulted in the inactivation of ERK1/2, whereas the phosphorylation of Akt at Thr308 and Ser473 as a result of mTORC2 activation was not increased. These results indicate that 1000 nmol/L rapamycin has an impact on MAPK activation status, but not PI3K-Akt(Thr308) or mTORC2-Akt(Ser473).
Next, we sought to determine the mechanism behind the rapamycin-dependent activation of ERK by examining its upstream regulators. The Raf1/MEK1/2 pathway is essential for the activation of ERK. We observed that 1000 nmol/L rapamycin induced the phosphorylation of Raf1 and that the MEK1/2 inhibitor UO126 abrogated rapamycin-dependent ERK1/2 activation. These results demonstrate that 1000 nmol/L rapamycin leads to activation of the Raf1/MEK1/2/ERK pathway.
Owing to the high sensitivity of p70S6K to rapamycin inhibition, disrupting p70S6K-mediated negative feedback may limit the efficacy of rapamycin and its derivatives in cancer.26 The inhibition of p70S6K leads to increased ERK activation mediated by rapamycin, indicating that, in the rapamycin-mediated process of MAPK activation, p70S6K is essential. Pharmacological inhibition of PI3K was subsequently performed in order to explore its role in rapamycin signaling to MAPK. PI3K inhibition by LY294002 suppressed rapamycin-mediated ERK activation. The results reveal that 1000 nmol/L rapamycin led to Raf1/MEK1/2/ERK pathway activation through the p70S6K-PI3K-mediated feedback loop in mesangial cells.
However, our problem is that rapamycin activates negative feedback only at a higher dose of 1000 nmol/L, but not at low doses (1–100 nmol/L), which contrasts with previous studies in cancer cells.21 There are two possible reasons for these differences. One is that there are differences in the signaling responses among different cell types. For example, the signaling pathways of tumor cells differ from those of normal cells, with tumor cells being more sensitive to stimulation by low-dose rapamycin. On the other hand, it may be that other undetected signaling pathways are activated in the tumor cells. Efeyan and Sabatini27 reported that, although most studies have focused on S6K1, it is also conceivable that mTORC1 inhibition may play roles not only through this target. For example, mTORC1, 2, AKT and deptor and p53, Pten and mTOR may also play an important role. However, the specific mechanisms of action still need further experimentation and discussion.
Rapamycin is generally used at low doses in a clinical context. We once thought that high doses of it may cause cell death, limiting its dosage. However, when we conducted cell experiments to explore the drug concentration and toxicity, we found that 1000 nM rapamycin did not exert obvious cytotoxicity and that its ability to block p70S6k phosphorylation was stronger, while the MTT assay showed that its inhibitory effect on cell proliferation was not enhanced. This study was established mainly to study this issue, so it has certain limitations. However, our work suggests that rapamycin is safer and more effective in the treatment of mesangial proliferative glomerular disease than in the treatment of cancer that appears to be resistant to mTOR inhibitors.
Conclusions
In conclusion, this study demonstrated that high-dose rapamycin leads to ERK1/2 activation through the p70S6K/PI3K/MAPK feedback loop in rat mesangial cells, thus reducing the inhibitory effect of rapamycin on mesangial cell proliferation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant No. 81500529, 81500518), The Applied Basic Research Program of Shanxi Province (No. 201901D111187, 201901D111188), and The Doctoral Startup Research Fund of Shanxi Medical University (No. 03201403).
ORCID iDs: Sijia Chang  https://orcid.org/0000-0002-9602-073X
https://orcid.org/0000-0002-9602-073X
Yanhong Wang  https://orcid.org/0000-0002-2306-5355
https://orcid.org/0000-0002-2306-5355
References
- 1. Wang W, Chan YH, Lee W, et al. (2001) Effect of rapamycin and FK506 on mesangial cell proliferation. Transplantation Proceedings 33(1–2): 1036–1037. [DOI] [PubMed] [Google Scholar]
- 2. Nagai K, Tominaga T, Ueda S, et al. (2017) Mesangial cell mammalian target of rapamycin complex 1 activation results in mesangial expansion. Journal of the American Society of Nephrology 28(10): 2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamouza H, Chemouny JM, Raskova Kafkova L, et al. (2012) The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney International 82(12): 1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu L, Hu X, Cai GY, et al. (2012) High glucose-induced hypertrophy of mesangial cells is reversed by connexin43 overexpression via PTEN/Akt/mTOR signaling. Nephrology Dialysis Transplantation 27(1): 90–100. [DOI] [PubMed] [Google Scholar]
- 5. Saxton RA, Sabatini DM. (2017) mTOR signaling in growth, metabolism, and disease. Cell 168(6): 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ersahin T, Tuncbag N, Cetin-Atalay R. (2015) The PI3K/AKT/mTOR interactive pathway. Molecular BioSystems 11(7): 1946–1954. [DOI] [PubMed] [Google Scholar]
- 7. Arriola Apelo SI, Lamming DW. (2016) Rapamycin: An inhibition mTOR of aging emerges from the soil of Easter Island. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 71(7): 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tian J, Wang Y, Zhou X, et al. (2014) Rapamycin slows IgA nephropathy progression in the rat. American Journal of Nephrology 39(3): 218–229. [DOI] [PubMed] [Google Scholar]
- 9. Filippone SM, Samidurai A, Roh SK, et al. (2017) Reperfusion therapy with rapamycin attenuates myocardial infarction through activation of AKT and ERK. Oxidative Medicine and Cellular Longevity 2017;4619–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen XG, Liu F, Song XF, et al. (2010) Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Molecular Carcinogenesis 49(6): 603–610. [DOI] [PubMed] [Google Scholar]
- 11. Soares HP, Ni Y, Kisfalvi K, et al. (2013) Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLoS One 8(2): e57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domínguez-Calderón A, Ávila-Flores A, Ponce A, et al. (2016) ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Molecular Biology of the Cell 27(10): 1581–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han F, Xue M, Chang Y, et al. (2017) Triptolide suppresses glomerular mesangial cell proliferation in diabetic nephropathy is associated with inhibition of PDK1/Akt/mTOR pathway. International Journal of Biological Sciences 13(10): 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ravichandran K, Zafar I, Ozkok A, et al. (2015) An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrology Dialysis Transplantation 30(1): 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stratakis S, Stylianou K, Petrakis I, et al. (2013) Rapamycin ameliorates proteinuria and restores nephrin and podocin expression in experimental membranous nephropathy. Clinical & Developmental Immunology 2013: 941893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hochegger K, Jansky GL, Soleiman A, et al. (2008) Differential effects of rapamycin in anti-GBM glomerulonephritis. Journal of the American Society of Nephrology 19(8): 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kramer S, Wang-Rosenke Y, Scholl V, et al. (2008) Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. American Journal of Physiology-Renal Physiology 294(2): F440–F449. [DOI] [PubMed] [Google Scholar]
- 18. Tian J, Wang Y, Guo H, et al. (2015) The Akt/mTOR/p70S6K pathway is activated in IgA nephropathy and rapamycin may represent a viable treatment option. Experimental and Molecular Pathology 99(3): 435–440. [DOI] [PubMed] [Google Scholar]
- 19. Rodrigues JC, Haas M, Reich HN. (2017) IgA nephropathy. Clinical Journal of the American Society of Nephrology 12(4): 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ni Y, Wang L, Zhang J, et al. (2015) PKD1 is downregulated in non-small cell lung cancer and mediates the feedback inhibition of mTORC1-p70S6K1 axis in response to phorbol ester. The International Journal of Biochemistry & Cell Biology 60: 34–42. [DOI] [PubMed] [Google Scholar]
- 21. Carracedo A, Ma L, Teruya-Feldstein J, et al. (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of Clinical Investigation 118(9): 3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang J, Kalogerou M, Samsel PA, et al. (2015) Renal tumours in a Tsc2(+/-) mouse model do not show feedback inhibition of Akt and are effectively prevented by rapamycin. Oncogene 34(7): 922–931. [DOI] [PubMed] [Google Scholar]
- 23. Dibble CC, Cantley LC. (2015) Regulation of mTORC1 by PI3K signaling. Trends in Cell Biology 25(9): 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galoian K, Temple HT, Galoyan A. (2012) mTORC1 inhibition and ECM-cell adhesion-independent drug resistance via PI3K-AKT and PI3K-RAS-MAPK feedback loops. Tumour Biology 33(3): 885–890. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Kim SG, Blenis J. (2014) Rapamycin: One drug, many effects. Cell Metabolism 19(3): 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fruman DA, Chiu H, Hopkins BD, et al. (2017) The PI3K pathway in human disease. Cell 170(4): 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Efeyan A, Sabatini DM. (2010) mTOR and cancer: Many loops in one pathway. Curr Opin Cell Biol 22(2): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]