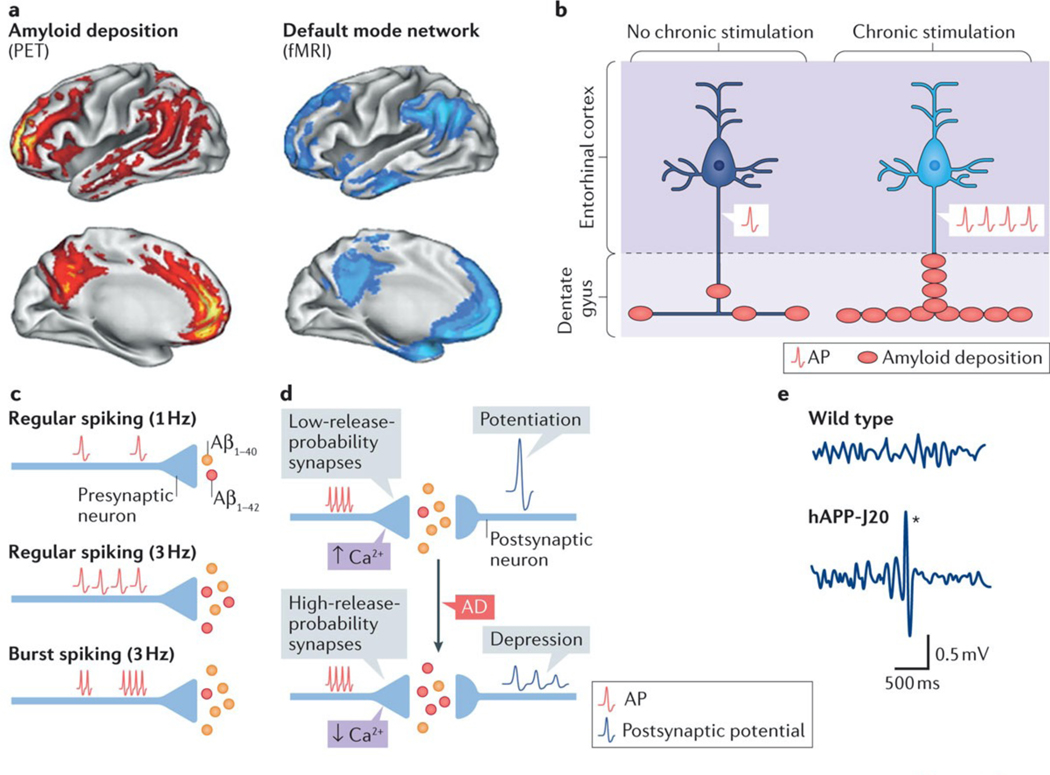
Figure 3 |. Neuronal activity regulates amyloid-β production and deposition.
a | Most patients with Alzheimer disease (AD) and some people without dementia have increased Pittsburg compound B-positive amyloid deposits in the brain243 (red). Amyloid deposits predominate in brain regions of the default mode network (blue), which shows deactivation deficits in AD (FIG. 2), suggesting a potential link between aberrant neuronal activity and amyloid deposition. b | In APP-A7 mice, chronic, optogenetic stimulation of pyramidal cells in the entorhinal cortex triggered epileptiform activity and increased amyloid deposition in the molecular layer of the dentate gyrus, directly supporting the notion that aberrant neuronal activity can promote amyloid deposition in vivo. c | At the synaptic level, an increase in the frequency of action potentials (APs) proportionally enhances amyloid-β1–42 (Aβ1–42) and amyloid-β1–40 (Aβ1–40) production. Compared with regular firing, burst firing reduces the Aβ1–42/Aβ1–40 ratio. d | Basal neurotransmitter release determines the ‘filter’ mode of synapses and regulates synaptic plasticity. The top panel indicates that excitatory synapses with lower release probability (high-pass-filter synapses) have greater presynaptic Ca2+ build-up, produce lower Aβ1–42/Aβ1–40 ratios, exhibit synaptic facilitation and primarily transfer potentiated responses. The bottom panel depicts synapses with higher release probability (low-pass-filter synapses), which have less presynaptic Ca2+ build-up, produce higher Aβ1–42/Aβ1–40 ratios and show synaptic depression as well as enhanced spike transfer. In AD, synapses may shift from high- to low-pass synaptic filtering. e | Electroencephalograhy recordings capture the combined electrical output of neuronal ensembles. In such recordings, most familial AD (FAD) mice, including hAPP-J20 (REF. 96), APP/PSEN1dE9 (REFS 102,103), Tg2576 (REF. 104), 5xFAD122, 3xTg-AD75, APP/TTA-EC105, APP/TTA-CaMKIIα106, and APP23 (REF. 107) mice (Supplementary information S1 (table)), show intermittent large-amplitude epileptiform discharges (denoted by the asterisk in hAPP-J20 mice; bottom panel), which provide evidence of network hypersynchrony. fMRI, functional MRI; PET, positron emission tomography. Part a is republished with permission of Society for Neuroscience, from Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory, Buckner, R. L. et al., 25, 34, 2005; permission conveyed through Copyright Clearance Center, Inc. Part e is adapted with permission from REF. 172, Cell Press/Elsevier.