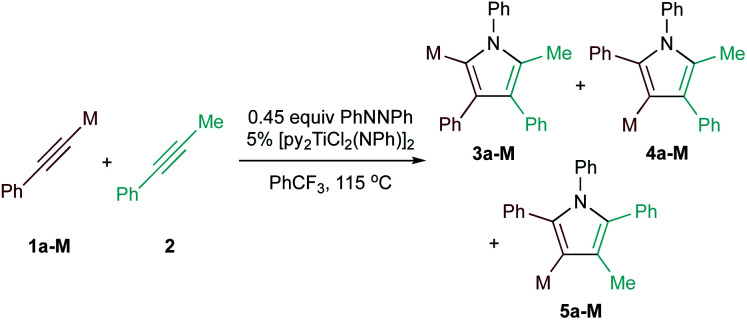
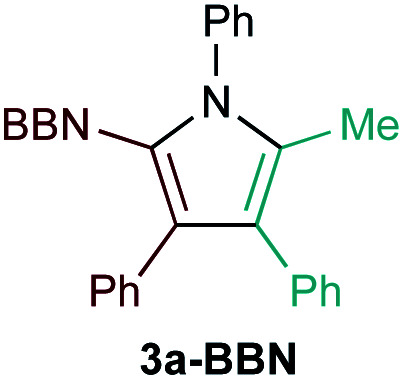
Examination of potential heteroatom-substituted alkyne partners in Ti-catalyzed [2 + 2 + 1] heterocouplinga.
| ||||
|---|---|---|---|---|
| Entry | X | Product | Yield | Selectivityb |
| 1d |
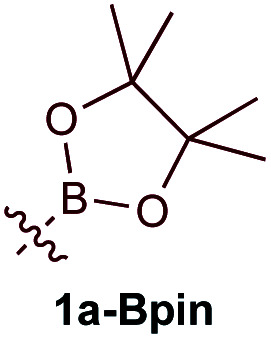
|
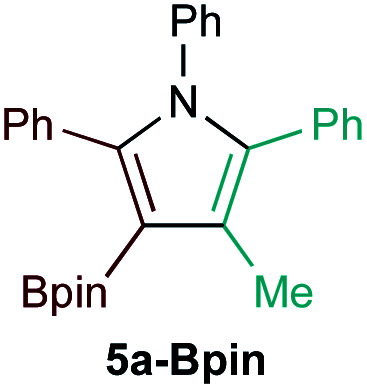
|
19% | 2.5 : 1 (1.1 : 1)c |
| 2e |
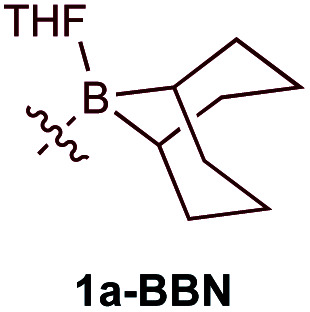
|
|
7% | 22.3 : 1 (12.5 : 1) |
| 3e |
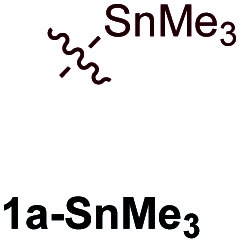
|
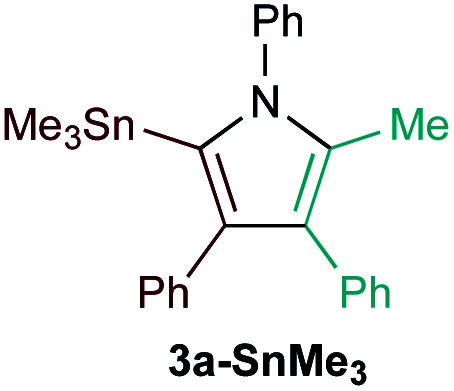
|
51% | 6.4 : 1 (4.5 : 1) |
| 4d |

|
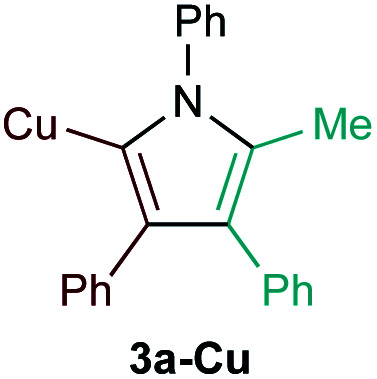
|
7% | n.d.f |
Conc. = 0.2 M.
Selectivity with respect to all heterocoupling pyrrole regioisomer products. Selectivity = 3a-M/(4a-M + 5a-M). In parenthesis: selectivity with respect to all possible pyrrole products. Selectivity in parenthesis = 3a-M/(4a-M + 5a-M + homocoupled products of 2).
Selectivities calculated for major heterocoupling product 5a-M instead of 3a-M.
t = 16 h.
t = 20 h.
Other pyrrole products cannot be quantified due to their low yield and peak overlapping.
