Optimization of the Ti-catalyzed [2 + 2 + 1] heterocoupling of 1a-BBN with 2a.
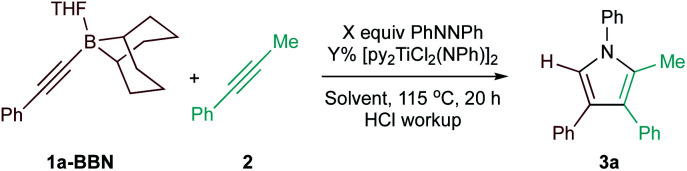
| ||||
|---|---|---|---|---|
| Entry | % [Ti]b | Solvent | [py] equiv.c | Yieldd (selectivitye) |
| 1 | 5 | PhCF3 | 0.2 | 7% (12.5 : 1) |
| 2 | 5 | C6D5Br | 0.2 | 22% (6.2 : 1) |
| 3 | 10 | C6D5Br | 0.4 | 74% (17.1 : 1) |
| 4 | 15 | C6D5Br | 0.6 | 65% (13.2 : 1) |
| 5 | 10 | PhCH3 | 0.4 | 67% (19.6 : 1) |
| 6 | 10 | PhCF3 | 0.4 | 55% (15.7 : 1) |
| 7 | 10 | PhOCH3 | 0.4 | 20% (9.6 : 1) |
| 8f | 10 | C6D5Br | 0.4 | 66% (22.7 : 1) |
Conc. = 0.2 M.
[PhNNPh] was adjusted coordinatingly to the change in [Ti] to keep the nitrene equivalent as 1, on basis of the relationship [nitrene] = [Ti] + 2[PhNNPh].
Total equivalent of pyridine in the reaction.
Yield determined by GC-FID.
Selectivity with respective to all possible pyrrole products.
t = 0.5 h.
