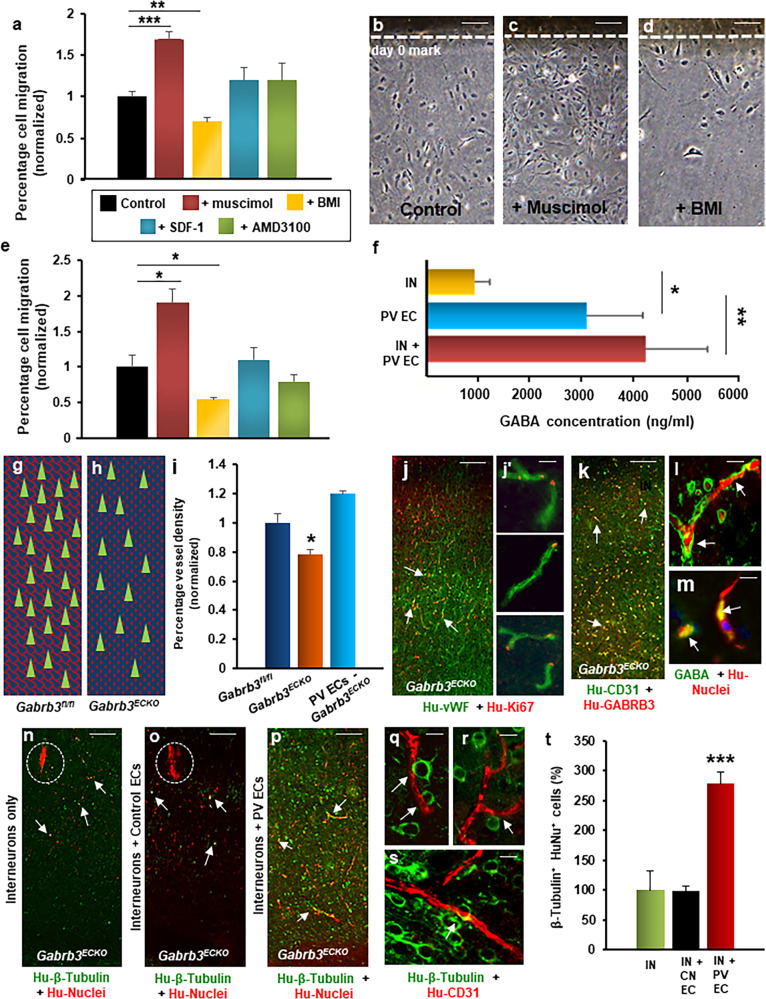
Fig. 4. Human periventricular endothelial cell-derived GABA regulates cell migration.
a–d Migration of periventricular endothelial cells in response to addition of GABAA receptor agonist muscimol (100 µM), GABAA receptor antagonist BMI (100 µM), chemokine SDF-1α (40 nM) and CXCR4 inhibitor AMD3100 (50 µM). a Quantitation of migration assay. Addition of muscimol resulted in higher percentage of cells migrating out, while addition of BMI resulted in less cell migration, compared to control (no chemicals added). The presence of SDF-1 or AMD3100 showed no significant effect on migration of periventricular endothelial cells (n = 5, mean ± SD, **P < 0.01, ***P < 0.001, Student’s t test). b–d Representative phase contrast images showing periventricular endothelial cell migration in control, +muscimol, and +BMI conditions. e Quantitation of interneuron migration when co-cultured with periventricular endothelial cells pre-treated with chemicals for 48 h. Interneurons co-cultured on muscimol-treated periventricular endothelial cells showed increased migration than those co-cultured on untreated periventricular endothelial cells (control), while interneurons co-cultured with BMI-treated periventricular endothelial cells showed decreased migration compared to control (n = 5, mean ± SD, *P < 0.05, Student’s t test). Treatment of periventricular endothelial cells with SDF or AMD3100 had no effect on interneuron migration. f Quantitation of GABA secretion measured by ELISA. GABA level was significantly higher in interneuron + periventricular endothelial cell co-culture and in periventricular endothelial cell-only population, compared to interneuron-only population. (n = 6, mean ± SD, *P < 0.05, **P < 0.01, Student’s t test). Schema depicting normal vasculature (red lattice pattern) and GABAergic interneurons (green triangles) in Gabrb3fl/fl cerebral cortex (g), while in Gabrb3ECKO cerebral cortex (h) there is both a vascular deficit (dotted red pattern) and a deficit in GABAergic interneurons (green triangles). i Quantification of vessel densities. Periventricular endothelial cell-transplanted Gabrb3ECKO mice showed significant rescue in vessel densities in the cerebral cortex when compared to Gabrb3ECKO mice (n = 6, mean ± SD, *P < 0.05, Student’s t test). Transplanted human periventricular endothelial cells undergo proliferation as shown by human vWF/Ki67 co-labeling (j; high magnification images shown in j′), and continue to express GABRB3 (k) and GABA (l, m) in vivo (white arrows). (n–o) Migration pattern of transplanted interneurons in somatosensory cortical region of transplanted Gabrb3ECKO brain. Grafted interneurons showed poor migratory ability (white arrows) and remained mostly near the grafted site (dotted white area) in interneuron-only transplanted brain (n) and interneuron + control endothelial cell co-transplanted brain (o). p In interneuron + periventricular endothelial cell co-transplanted brains, interneurons showed extensive migration and widespread distribution (white arrows). q–s Magnified images showing close interactions between transplanted interneurons and new vessels formed by human periventricular endothelial cells. t Quantitation of β-tubulin+/human nuclei+ neurons in cortex (including cingulate, motor, somatosensory and piriform cortical regions) of Gabrb3ECKO transplanted brain, 30 days after grafting. Significantly higher number of grafted neurons in interneuron + periventricular endothelial cell co-transplanted Gabrb3ECKO brains showed migration all over cortex, compared to those in interneuron-only or interneuron + control endothelial cell-transplanted brains. Data represent mean ± SD, (n = 20, ***P < 0.001, Student’s t test). Scale bar: b 100 μm (applies to c, d, j, k, n–p), l 50 µm (applies to j′ m, q, r, s). IN interneurons, CN EC control endothelial cells derived without GABA and WNT7A, PV ECs periventricular endothelial cells.