Fig. 1.
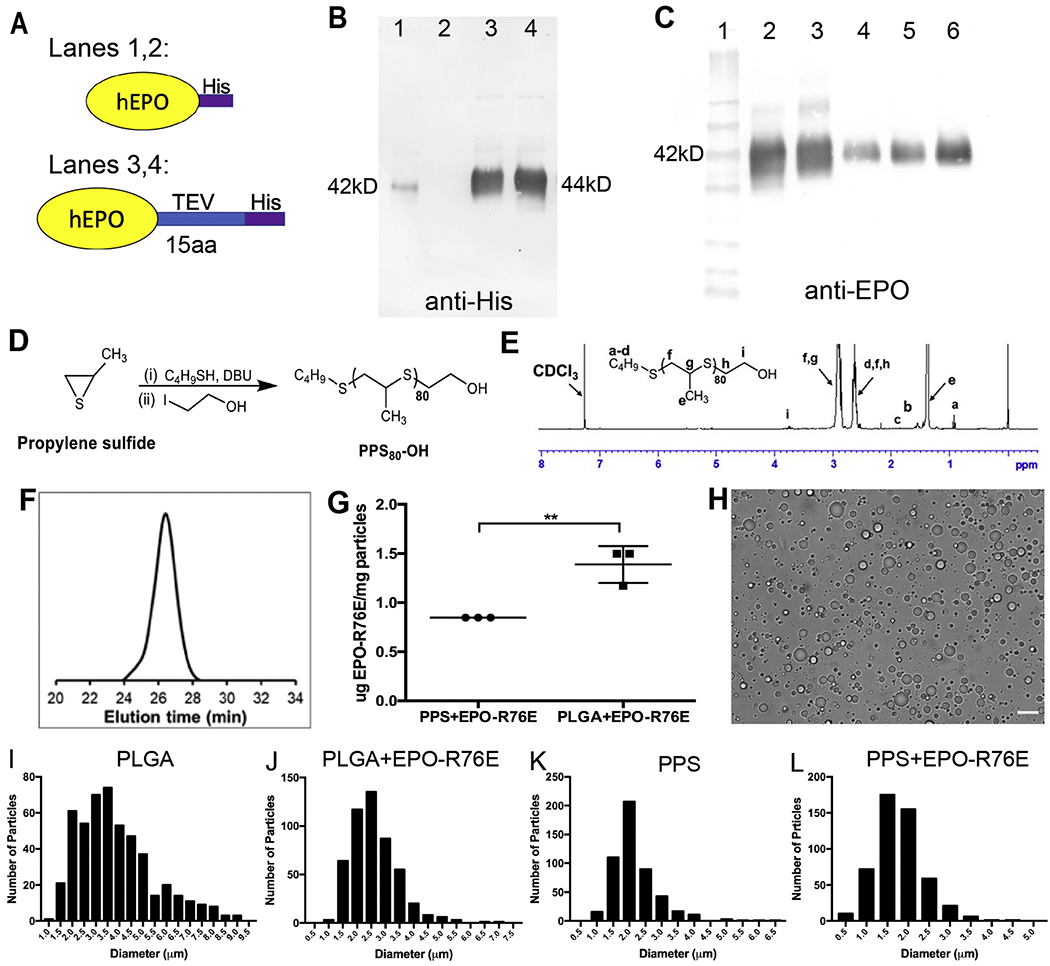
Production of EPO-R76E containing MPs. A) Schematic of two His-tagged human EPO-R76E (hEPO) that were produced. B) Detection of purified EPO-R76E using anti-His. Very little His-tagged wild-type EPO (Lane 1) and no His-tagged EPO-R76E (Lane 2) was detected. The addition of a TEV linker resulted in a large increase in the amount of detectable His-EPO (Lane 3) and His-EPO-R76E (Lane 4). Notably, both proteins are the same size. C) Detection of the TEV-His-tagged protein using anti-EPO and comparison to commercially available EPO. Lane 1: MW markers, Lane 2: wild-type EPO-TEV-His, Lane 3: EPO-R76E-TEV-His, Lanes 4–6: commercial EPO at 25 ng (4), 50 ng (5), and 100 ng (6). All were detectable with the EPO antibody and were the same size. D) Schematic for synthesis of PPS by anionic ring opening polymerization. E) 1H NMR spectra of PPS in CDCl3. F) GPC refractive index detector trace of PPS (PS80-OH) confirmed the formation of polymer. G) Quantification of Cy7 conjugated EPO-R76E loading into PLGA and PPS MPs. H) Representative brightfield microscopy image of unloaded PPS MPs. Scale bar represents 10 μm. I-L) Quantitative histograms demonstrating size distribution of unloaded and EPO-R76E loaded PLGA and PPS MPs. Note differences in axes between graphs.
