Fig. 1.
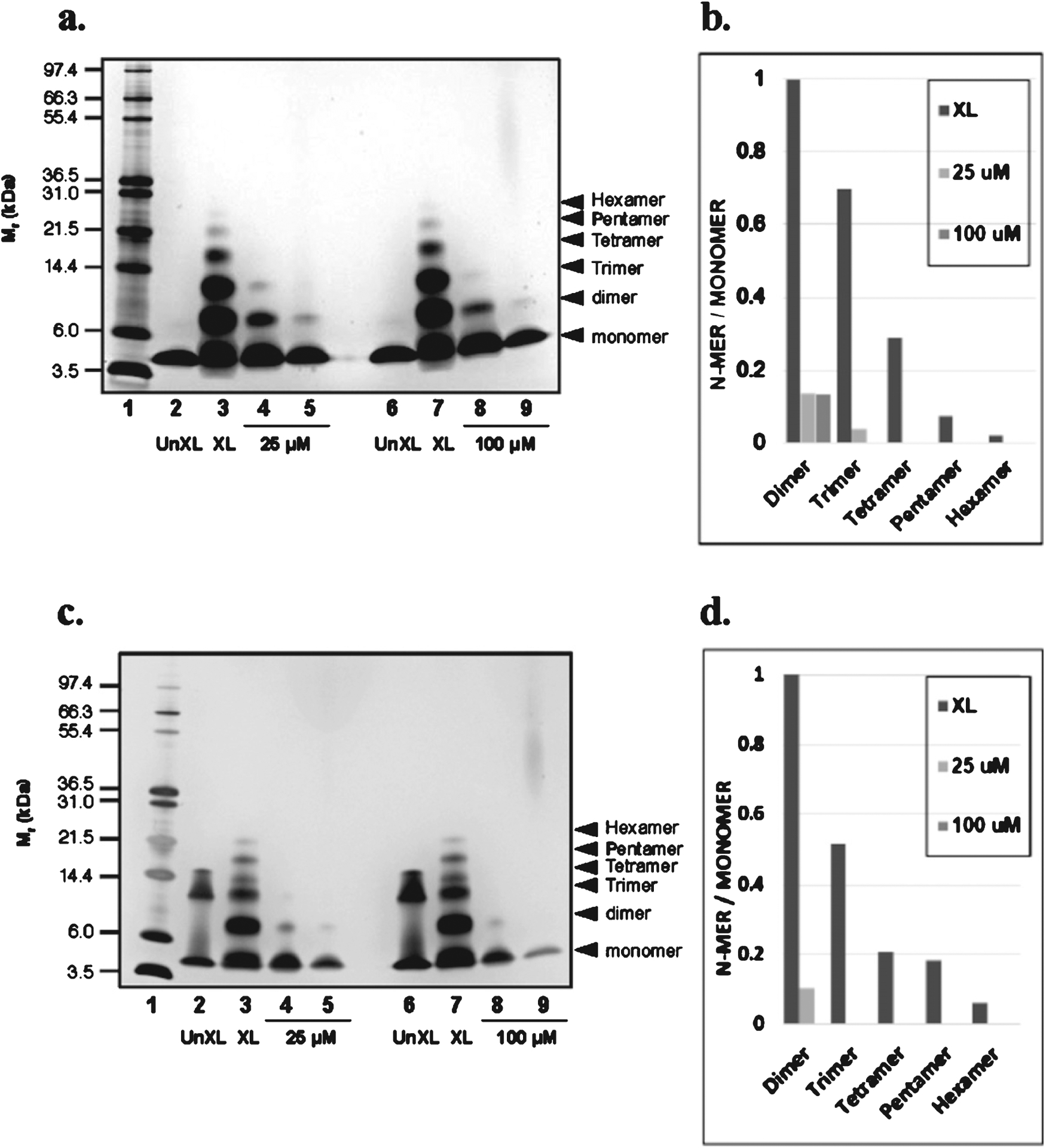
Oligopin attenuates oligomerization of Aβ peptides in vitro. SDS-PAGE of Aβ1–40 and Aβ1–42 in the presence or absence of oligopin following PICUP. (a) 25 μM Aβ1–40 and (c) Aβ1–42 were cross-linked in the presence or absence of 25 (1:1) or 100 (1:4) μM oligopin and the bands in subsequent SDS gels were visualized using silver staining. Lane 1: molecular weight; Lanes 2 and 6: UnXL, un-cross-linked Aβ1–40 or Aβ1–42; Lanes 3 and 7: XL, cross-linked Aβ1–40 or Aβ1–42; Lanes 5 and 9: Aβ1–40 or Aβ1–42 aggregated in the presence of 25 or 100 μM oligopin; the molecular weight of oligopin is based on the composition of dimer, trimer and tetramer of oligopin. A compound related to oligopin was used as a control to asses attenuation of Aβ1–40 or Aβ1–42 purposes relative to oligopin (lanes 4 and 8). For each lane that contains crosslinked samples (XL) in a and c, the ratios of the integrated densities of each oligomer to the monomer are shown in panels b and d, respectively. The gels are representative of three independent experiments.
