Abstract
PURPOSE
To present a summary of the treatment and follow-up recommendations for the biochemical recurrence in castration-sensitive prostate cancer (PCa) acquired through a questionnaire administered to 99 PCa experts from developing countries during the Prostate Cancer Consensus Conference for Developing Countries.
METHODS
A total of 27 questions were identified as related to this topic from more than 300 questions. The clinician's responses were tallied and presented in a percentage format. Topics included the use of imaging for staging biochemical recurrence, treatment recommendations for three different clinical scenarios, the field of radiation recommended, and follow-up. Each question had 5-7 relevant response options, including “abstain” and/or “unqualified to answer,” and investigated not only recommendations but also if a limitation in resources would change the recommendation.
RESULTS
For most questions, a clear majority (> 50%) of clinicians agreed on a recommended treatment for imaging, treatment scenarios, and follow-up, although only a few topics reached a consensus > 75%. Limited resources did affect several areas of treatment, although in many cases, they reinforced more stringent criteria for treatment such as prostate-specific antigen values > 0.2 ng/mL and STAMPEDE inclusion criteria as a basis for recommending treatment.
CONCLUSION
A majority of clinicians working in developing countries with limited resources use similar cutoff points and selection criteria to manage patients treated for biochemically recurrent castration-sensitive PCa.
INTRODUCTION
Prostate cancer (PCa) is the second most common cancer in men worldwide.1 Men's lifetime risk of diagnosis is 15%, whereas the lifetime mortality remains low at 3%, often over the age of 75.2 The treatment for localized PCa can vary greatly—ranging from active surveillance3-5 to active treatment with curative intent with radical prostatectomy (RP) or primary definitive radiotherapy (RT)—depending on its progression risks.3,6 Although it might be curative in many cases, approximately 30%-40% of men will develop a biochemical recurrence (BCR),7,8 which is defined by a rising serum prostate-specific antigen (PSA) following definitive local therapies (ie, RT or RP) without metastases detected by available imaging modalities. Even when there is no evidence or symptoms of a locally recurrent or metastatic disease, this BCR does indicate the recurrence of cancer and does not correlate with the patient's quality of life (QoL) or overall survival.9
CONTEXT
Key Objective
To generate a consensus on critical issues relevant to the treatment of biochemical recurrence (BCR) in castration-sensitive prostate cancer focused on developing countries.
Knowledge Generated
In a limited resource setting, patients with BCR should undergo chest computed tomography or X-ray, and computed tomography or magnetic resonance imaging of abdomen and pelvis and bone scans. For patients with BCR post-prostatectomy, the prostate-specific antigen level ≥ 0.2 mg/mL is the cutoff to initiate salvage therapy. The salvage therapy option for BCR post-prostatectomy should be radiation therapy with androgen deprivation therapy for 6 months. The salvage therapy option for BCR after local definitive treatment (radiotherapy and/or brachytherapy) should be salvage prostatectomy. If prostatectomy is not feasible, intermittent hormonal therapy with androgen deprivation therapy should be considered.
Relevance
The voting results presented in this document can be used to support the treatment of BCR in castration-sensitive prostate cancer in areas of limited resources lacking specific guidelines.
Clinicians are provided with several options to manage patients with BCR. The challenge relies on preventing or delaying the onset of metastatic disease and the resulting morbidity and mortality while also considering the negative impact on patients' QoL and avoidance of overtreating PCa of a low risk of clinical progression. As the incidence and burden of PCa steadily increases globally, its management also presents new challenges for healthcare systems,10 especially in regions of limited resources. Although the benefits of technological advances have offered improvements in detection, screening, treatment, and outcomes, there is a great need to better tailor treatment recommendations according to the individual risk of metastatic disease or death while also balancing the overall costs and burden for healthcare systems.10 The present study summarizes treatment and follow-up recommendations from a large panel of physicians working with PCa in developing countries for the recommended treatment and follow-up of patients presenting with BCR of castration-sensitive PCa—with and without the consideration of limited resources. It aims to provide a guideline that can be used in clinical practice and policy development by physicians or policymakers, especially in limited-resource settings.
METHODS
This study is part of a series of articles about the first global Prostate Cancer Consensus Conference for Developing Countries (PCCCDC). Full information about the conference, methods, and survey are described in an editorial submitted as another manuscript. The first global PCCCDC was organized around state-of-the-art lectures and presentations and it discussed evidence relevant to 12 key topics and subtopics related to the management of PCa in general and in limited-resource regions (screening, diagnosis, staging tools, treatment, and follow-up for various stages of cancer). Four polling sessions were scheduled during the 2-day conference for panelists to respond to questions regarding these topics. Only physicians who participated in all four sessions were included in the final consensus results.
The full panel for this consensus paper consisted of 99 multidisciplinary cancer physicians, including urologists, medical oncologists, radiation oncologists, radiologists, and pathologists from developing countries in Latin America, Africa, Middle East Asia, and Eastern Europe. The panel members were selected based on their special interest in PCa, recent work in this field, and attendance at the first global PCCCDC.
The questionnaire was developed by a panel of seven experts to provide relevant real-world physician recommendations for nonfrail patients (as defined by Eastern Cooperative Oncology Group performance status 0-2) and for patients with prostate adenocarcinoma (unless otherwise stated). A total of 321 questions were constructed to investigate (1) screening, (2) diagnosis, (3) staging tools, (4) treatment, and (5) follow-up of PCa and the impact of limited resources on those treatment recommendations by the panelists. Following each question, there were five to seven relevant answers, including two nonanswers (“abstain” and “unqualified to answer”). The two nonanswers were provided for quality control and allowed for physicians to opt out of questions that they may not contend within their specific specialty. Unless stated otherwise, it is assumed that for the specific recommendation (the type of surgery, type of RT, and drug), therapies are approved and available, no treatment contraindications exist, and no clinical trial is currently in progress. For the questions that refer to an area of limited resources, the recommendations consider cost-effectiveness and the possible therapies with easier and greater access. Each question was deemed consensus if 75% or more of the full panel selected a particular answer. Their answers are annotated and discussed in the following sections where screening, diagnosis, and staging tools, and treatment for the topics stated are addressed. There was no patient advocate present at the conference. The complete methodology of PCCCDC, including the elaboration process of the questionnaires to guide the panelists, the design of voting sessions, and consensus criteria, is presented in the editorial and is valid for all the papers in this issue.
Staging
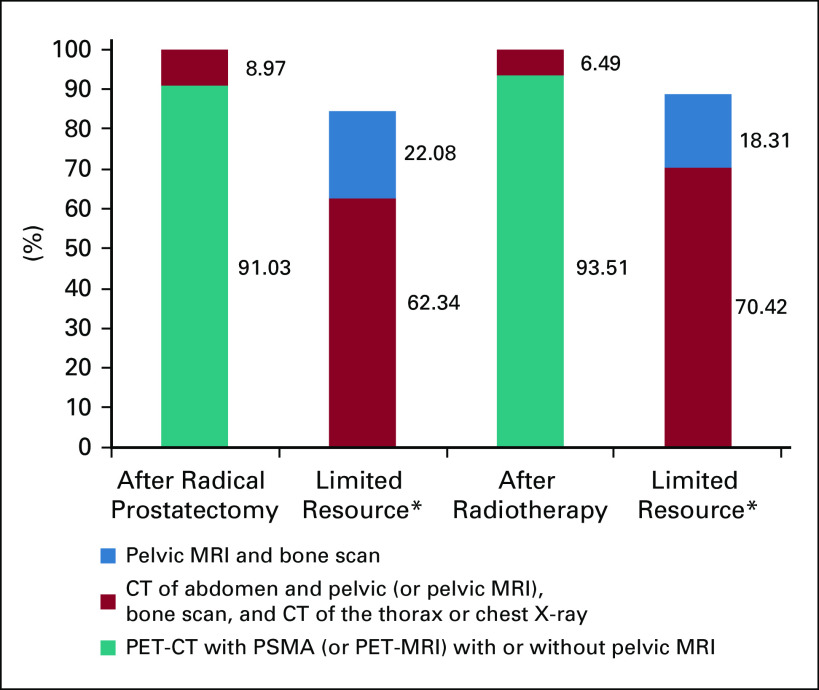
The staging recommendations are presented in a previously published work of this project (REF—in press) and are summarized in Figure 1. There was an overwhelming consensus by the clinicians for the use of positron emission tomography (PET) and/or computed tomography (CT) with prostate-specific membrane antigen (PET and/or CT-PSMA) or PET-magnetic resonance imaging (MRI) after both RP and RT (91.03% and 93.51%, respectively). In a limited resource setting, with no access to PET and/or CT-PSMA (or PET and/or MRI-PSMA), the clinicians recommended the combined use of CT of the abdomen and pelvis (or pelvic MRI), a bone scan, and a CT of the thorax or chest X-ray both after RP and after RT, as can be seen in Figure 1.
FIG 1.
The use of imaging in staging for castration-sensitive PCa with BCR with or without limited resources. BCR, biochemical recurrence; CT, computed tomography; MRI, magnetic resonance imaging; PCa, prostate cancer; PET-CT, positron emission tomography computer tomography; PSMA, prostate-specific membrane antigen.
Because of its low contrast resolution, CT is no longer recommended for detecting locoregional relapse of PCa after RP or RT.11 Modern PET and/or CT imaging techniques provide better sensitivity for metastasis detection, especially in BCR with low PSA levels, than conventional imaging such as bone scan, CT, and MRI. Recent literature reviews suggest that PSMA PET and/or CT is superior in detecting BCR; however, the impact of its increased sensitivity on patient survival is unknown and further research is required. These new tracers pose a challenge for accessibility, and further validation from clinical trials is needed to evaluate their benefit in routine clinical practice.12
Treatment Recommendations
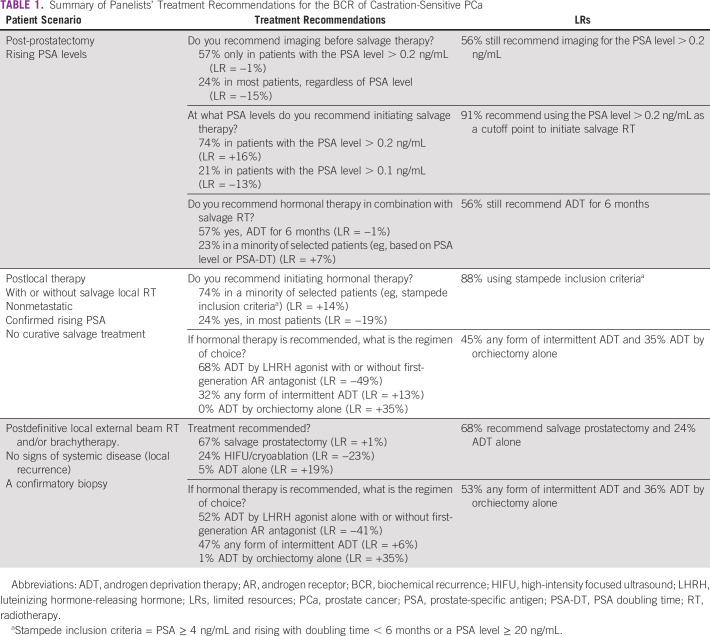
Three different patient scenarios were posed to the clinicians for their treatment recommendations in case there is BCR exhibited by rising PSA levels after primary curative treatment. These scenarios are presented in Table 1 with a summary of the main findings. For the sake of clarity and ease of interpretation, not all the answer categories are presented—only those that had been chosen by a significant number of physicians are presented. Therefore, the category total presented does not culminate to the full 100%. The complete data with all the answers presented are given in the Data Supplement. The impact that limited resources had on the question is denoted as a gray LR = with a+ or − and a numerical value. This represents the change in percentage of experts making this recommendation when limited resources were considered (ie, 57% with LR = −1%, which means 57% − 1% = 56% in limited resources). The main impact of limited resources is highlighted in the column to the left of treatment recommendations. As can be seen in Table 1, there was little consensus as defined by reaching more than 75% of physicians responding with the same recommendation—although, in general, there were clear preferences of the majority (> 50%) of respondents reaching near-consensus levels.
TABLE 1.
Summary of Panelists' Treatment Recommendations for the BCR of Castration-Sensitive PCa
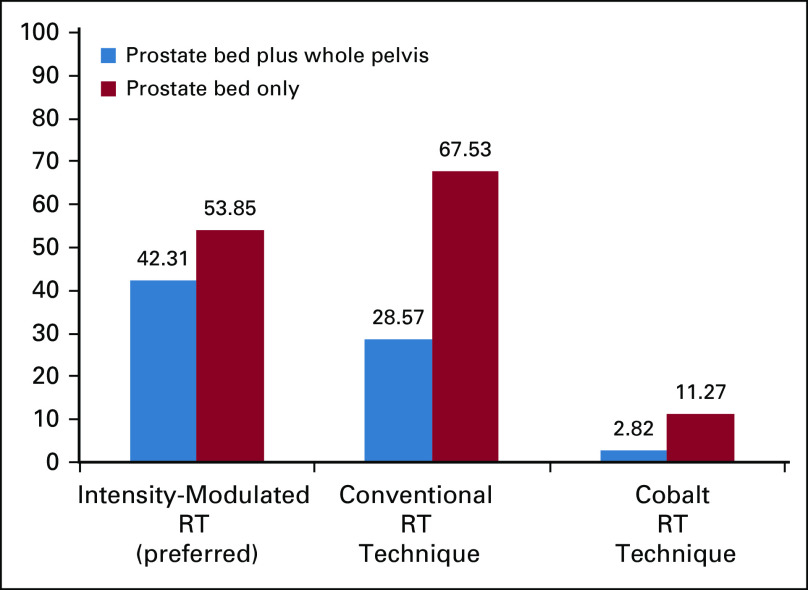
In patients, post-prostatectomy with rising PSA levels, imaging is recommended (57%) before salvage RT if their PSA value is greater than 0.2 ng/mL, with 74% also recommending 0.2 ng/mL as the cutoff to initiate salvage therapy (91% in limited-resource scenarios). With limited resources, 56% of the panel still recommended imaging before salvage therapy in those with a PSA value > 0.2 ng/mL, with fewer recommending imaging in the majority of patients regardless of the PSA level (24%). More than half of the panelists (57%) recommended hormonal therapy with androgen deprivation therapy (ADT) for 6 months during salvage radiation, whereas 23% of panelists recommended it only for a minority of selected patients according to criteria, such as the PSA level ≥ 0.5 ng/mL and/or the PSA doubling time ≤ 6 months. For clinicians practicing in limited-resource settings, the conduct does not vary regarding their decision for recommending hormonal therapy in combination with salvage RT. The panelist-recommended modalities for salvage RT are shown in Figure 2.
FIG 2.
Modality recommendations for salvage RT. RT, radiation therapy.
In patients with nonmetastatic disease with a confirmed rising PSA level, postlocal therapy with or without local salvage therapy, and no curative salvage therapy treatment option, the majority of experts (74%) recommend hormonal treatment for a fraction of selected patients, with only 24% suggesting initiation of ADT for a majority of patients. In a limited-resource setting, 88% of the panelists used these criteria (rising PSA level) to recommend ADT. In cases where hormonal therapy is recommended, 68% of panelists preferred ADT with luteinizing hormone-releasing hormone (LHRH) agonist with or without first-generation antiandrogen, although the recommendation for this option in a limited-resource scenario was drastically reduced to 19%, with 45% opting for any form of intermittent ADT and 35% opting for ADT with orchiectomy alone.
In patients with a local recurrence (no signs of systemic disease) with a confirmatory biopsy where definitive local external beam RT and/or brachytherapy had been used, salvage prostatectomy was the recommended course of treatment by 68% and 67% of the panelists with and without limited resources, respectively. Salvage high-intensity focused ultrasound was suggested by almost a quarter (24%) of panelists if this resource is available. Hormonal therapy in this patient scenario was split near evenly between any form of intermittent ADT (47%) and 52% preferring ADT with LHRH agonist with or without first-generation antiandrogen. In limited-resource environments, 53% recommended any form of intermittent ADT and 36% recommended ADT with orchiectomy alone.
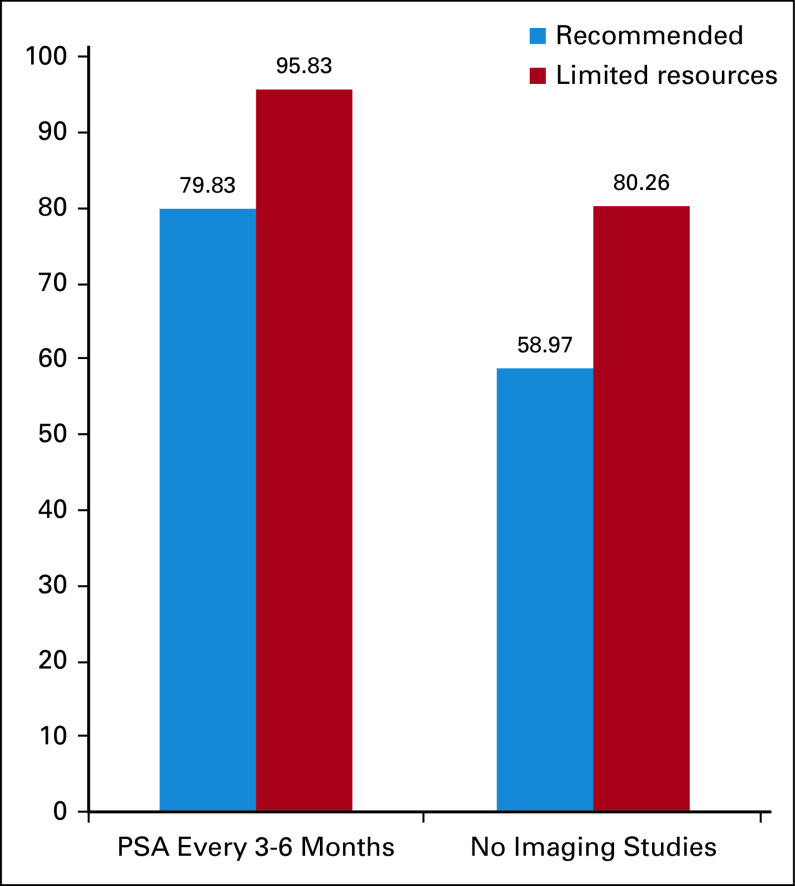
Following-up with PSA measurements every 3-6 months was recommended by 80% of the panelists, 96% in the case of limited resources in patients having undergone a salvage local therapy (after initial prostatectomy and/or RT) with curative intent with BCR and no evidence of disease. Although most clinicians (59%) did not recommend imaging in follow-up for these cases, 30% recommended in only a few cases. In limited resources, 80% did not recommend imaging, and 16% recommended in only a few cases (Fig 3). As such, there was a little consensus of the type of imaging study that would be recommended with 50% (and 76%—limited resources) only in the case of symptoms—see the Data Supplement.
FIG 3.
Recommendation for follow-up after a salvage local therapy after initial prostatectomy and/or radiotherapy. PSA, prostate-specific antigen.
DISCUSSION
Although a rising PSA level does universally precede metastasis and PCa-specific death, it is important to establish that BCR is not a surrogate for PCa-specific mortality or overall survival. A rising PSA level may predate local recurrence or metastasis by 7-8 years on average.13,14 This presents a different set of challenges for clinicians in developing countries where healthcare systems are challenged by decreased access to care, scarcity of technological advancements, overcrowded facilities, and large waiting times for specialists consultation, increasing the chances that patients may not be timely and accurately diagnosed and treated. Most of these recommendations failed to reach full consensus, defined as the agreement of 75% of experts recommending the same course of treatment because of the range of scenarios that clinicians face in real-world practice with a difficult healthcare system. Nevertheless, there were clear majorities in virtually all categories of questions for different clinical scenarios.
In this context, for patients with rising PSA value > 0.2 ng/mL post-prostatectomy, the expert panel recommends salvage RT to prostatic bed with LHRH agonist for 6 months. Considering the possibility of limited resources in developing countries, if the use of LHRH agonist is not possible, it is reasonable to use exclusive RT with a 3D conventional technique in this situation. Corroborating this recommendation, there are data showing five-year progression-free survival of 87%, 70%, and 47% for PSA levels < 0.3, 0.3-0.7, and > 0.7 ng/mL (P < .001), respectively, with exclusive salvage RT for the prostatic bed.15 For patients with rising PSA post-RT, our expert panel recommends salvage prostatectomy. However, the difficulties and high morbidity associated with salvage prostatectomy are well-known, especially in places without centers of expertise and limited resources. In this situation, with the PSA level > 4 ng/mL and the PSA doubling time < 6 months or the PSA level > 20 ng/mL (STAMPEDE criteria), intermittent ADT with LHRH agonist is recommended. If LHRH is unavailable, orchiectomy is an acceptable option. In the case of a rising PSA without any STAMPEDE criteria, observation is a good option.
The current study provides the first discussion and adaptation of not only best practices but also adaptations of those recommendations because of limited resources. This offers a far more practical application of expert recommendations for a large portion of the world in which medicine is practiced under limitations. While discussing optimal treatments, there is not a healthcare system in the world that is perfect or not affected by financial restraints, although some more significantly than others. Treatment decisions consistently need to be contextualized within the overall health and prognosis of the patient and their QoL and the overall healthcare system.
Fernando S. M. Monteiro
Consulting or Advisory Role: Bristol Myers Squibb, Roche, MSD Oncology, Janssen
Speakers' Bureau: Janssen, MSD Oncology, Ipsen
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: Janssen, Roche, Bristol Myers Squibb
Fabio A. Schutz
Employment: Ipsen (I)
Consulting or Advisory Role: Bayer, Janssen Oncology, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Pfizer, Astellas Pharma, AstraZeneca
Speakers' Bureau: Janssen Oncology, Astellas Pharma, Bayer, Pfizer, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche
Research Funding: Janssen Oncology
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Astellas Pharma, Janssen-Cilag
Igor A. P. Morbeck
Honoraria: Janssen-Cilag, BMS Brazil, AstraZeneca, MSD Oncology, Astellas Pharma
Consulting or Advisory Role: BMS Brazil, Janssen-Cilag, Takeda
Travel, Accommodations, Expenses: Astellas Pharma, BMS Brazil
Diogo A. Bastos
Honoraria: MSD, Roche, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, AstraZeneca, Bayer
Consulting or Advisory Role: Roche, Bayer, Janssen-Cilag, MSD Oncology
Research Funding: Janssen-Cilag (Inst), Pfizer (Inst), Astellas Pharma (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Bayer
Leonardo A. G. A. Costa
Honoraria: Janssen Oncology, Astellas Pharma, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology, Boehringer Ingelheim, Astellas Pharma, AstraZeneca
Manuel C. Maia
Consulting or Advisory Role: AstraZeneca, Janssen Oncology
Speakers' Bureau: Janssen Oncology, AstraZeneca, Bayer, MSD Oncology, Pfizer, Astellas Pharma
Expert Testimony: MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology
Stenio de Cassio Zequi
Consulting or Advisory Role: Pfizer, Astellas Brazil
Speakers' Bureau: Pfizer, Astellas Pharma, Bayer, Janssen, Astra Zeneca Brazil
Karine M. da Trindade
Honoraria: BMS Brazil, Janssen-Cilag, MSD Oncology
Consulting or Advisory Role: MSD Oncology, Janssen-Cilag, Astellas Pharma
Research Funding: BMS Brazil, Roche/Genentech, MSD Oncology, Janssen-Cilag
Travel, Accommodations, Expenses: Janssen-Cilag, BMS Brazil, Ipsen
Lucas V. dos Santos
Stock and Other Ownership Interests: Merck Sharp & Dohme, Eisai, Fleury Group
Honoraria: BMS Brazil, United Medical, Roche/Genentech
Consulting or Advisory Role: Lilly, Bristol Myers Squibb, MSD, Roche/Genentech
Speakers' Bureau: BMS Brazil, United Medical
Research Funding: Roche/Genentech (Inst), Janssen Oncology (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Amgen (Inst), Boston Scientific (Inst), Takeda (Inst), BMS Brazil (Inst), MSD (Inst), Exelixis (Inst)
Diogo A. R. da Rosa
Honoraria: Roche
Consulting or Advisory Role: Janssen-Cilag, Bristol Myers Squibb, AstraZeneca, Astellas Pharma
Speakers' Bureau: AstraZeneca, Dr Reddy's Laboratories, Roche, MSD Oncology, BMS Brazil
Travel, Accommodations, Expenses: Janssen-Cilag, Roche, BMS Brazil, Ipsen
Francisco J. Orlandi
Honoraria: Roche/Genentech
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Bristol Myers Squibb, MSD Oncology, Pfizer, Novartis, Sanofi
Speakers' Bureau: AstraZeneca/MedImmune, Roche
Research Funding: AstraZeneca/MedImmune, Amgen, Genentech/Roche, Boehringer Ingelheim, Astellas Medivation, MSD Oncology, Bristol Myers Squibb, Celltrion, Pfizer, mAbxience, Nektar, Sanofi
Travel, Accommodations, Expenses: MSD Oncology, Genentech/Roche
Fernando N. G. de Oliveira
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology, Astellas Pharma, MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, MSD Oncology
Andrey Soares
Honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi, Roche, MSD
Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol Myers Squibb (Inst)
Research Funding: Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Astellas Pharma, Bristol Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, MSD
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Fernando S. M. Monteiro, Igor A. P. Morbeck, Fernando V. de Padua, Leonardo A. G. A. Costa, William C. Nahas, Robson Ferrigno, Diogo A. R. da Rosa, Andrey Soares
Administrative support: William C. Nahas, Lucas V. dos Santos, Andrey Soares
Provision of study materials or patients: Fernando S. M. Monteiro, Lucas V. dos Santos, Juan P. Sade, Fernando N. G. de Oliveira
Collection and assembly of data: Fabio A. Schutz, Fernando V. de Padua, Leonardo A. G. A. Costa, Manuel C. Maia, Jose A. Rinck Jr, Stenio de Cassio Zequi, Karine M. da Trindade, Wladimir Alfer Jr, Lucas V. dos Santos, Diogo A. R. da Rosa, Juan P. Sade, Francisco J. Orlandi, Fernando N. G. de Oliveira, Andrey Soares
Data analysis and interpretation: Fernando S. M. Monteiro, Fabio A. Schutz, Diogo A. Bastos, Fernando V. de Padua, Jose A. Rinck Jr, Stenio de Cassio Zequi, Lucas V. dos Santos, Diogo A. R. da Rosa, Francisco J. Orlandi, Andrey Soares
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Fernando S. M. Monteiro
Consulting or Advisory Role: Bristol Myers Squibb, Roche, MSD Oncology, Janssen
Speakers' Bureau: Janssen, MSD Oncology, Ipsen
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: Janssen, Roche, Bristol Myers Squibb
Fabio A. Schutz
Employment: Ipsen (I)
Consulting or Advisory Role: Bayer, Janssen Oncology, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Pfizer, Astellas Pharma, AstraZeneca
Speakers' Bureau: Janssen Oncology, Astellas Pharma, Bayer, Pfizer, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche
Research Funding: Janssen Oncology
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Astellas Pharma, Janssen-Cilag
Igor A. P. Morbeck
Honoraria: Janssen-Cilag, BMS Brazil, AstraZeneca, MSD Oncology, Astellas Pharma
Consulting or Advisory Role: BMS Brazil, Janssen-Cilag, Takeda
Travel, Accommodations, Expenses: Astellas Pharma, BMS Brazil
Diogo A. Bastos
Honoraria: MSD, Roche, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, AstraZeneca, Bayer
Consulting or Advisory Role: Roche, Bayer, Janssen-Cilag, MSD Oncology
Research Funding: Janssen-Cilag (Inst), Pfizer (Inst), Astellas Pharma (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Bayer
Leonardo A. G. A. Costa
Honoraria: Janssen Oncology, Astellas Pharma, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology, Boehringer Ingelheim, Astellas Pharma, AstraZeneca
Manuel C. Maia
Consulting or Advisory Role: AstraZeneca, Janssen Oncology
Speakers' Bureau: Janssen Oncology, AstraZeneca, Bayer, MSD Oncology, Pfizer, Astellas Pharma
Expert Testimony: MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology
Stenio de Cassio Zequi
Consulting or Advisory Role: Pfizer, Astellas Brazil
Speakers' Bureau: Pfizer, Astellas Pharma, Bayer, Janssen, Astra Zeneca Brazil
Karine M. da Trindade
Honoraria: BMS Brazil, Janssen-Cilag, MSD Oncology
Consulting or Advisory Role: MSD Oncology, Janssen-Cilag, Astellas Pharma
Research Funding: BMS Brazil, Roche/Genentech, MSD Oncology, Janssen-Cilag
Travel, Accommodations, Expenses: Janssen-Cilag, BMS Brazil, Ipsen
Lucas V. dos Santos
Stock and Other Ownership Interests: Merck Sharp & Dohme, Eisai, Fleury Group
Honoraria: BMS Brazil, United Medical, Roche/Genentech
Consulting or Advisory Role: Lilly, Bristol Myers Squibb, MSD, Roche/Genentech
Speakers' Bureau: BMS Brazil, United Medical
Research Funding: Roche/Genentech (Inst), Janssen Oncology (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Amgen (Inst), Boston Scientific (Inst), Takeda (Inst), BMS Brazil (Inst), MSD (Inst), Exelixis (Inst)
Diogo A. R. da Rosa
Honoraria: Roche
Consulting or Advisory Role: Janssen-Cilag, Bristol Myers Squibb, AstraZeneca, Astellas Pharma
Speakers' Bureau: AstraZeneca, Dr Reddy's Laboratories, Roche, MSD Oncology, BMS Brazil
Travel, Accommodations, Expenses: Janssen-Cilag, Roche, BMS Brazil, Ipsen
Francisco J. Orlandi
Honoraria: Roche/Genentech
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Bristol Myers Squibb, MSD Oncology, Pfizer, Novartis, Sanofi
Speakers' Bureau: AstraZeneca/MedImmune, Roche
Research Funding: AstraZeneca/MedImmune, Amgen, Genentech/Roche, Boehringer Ingelheim, Astellas Medivation, MSD Oncology, Bristol Myers Squibb, Celltrion, Pfizer, mAbxience, Nektar, Sanofi
Travel, Accommodations, Expenses: MSD Oncology, Genentech/Roche
Fernando N. G. de Oliveira
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology, Astellas Pharma, MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, MSD Oncology
Andrey Soares
Honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi, Roche, MSD
Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol Myers Squibb (Inst)
Research Funding: Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Astellas Pharma, Bristol Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, MSD
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Wever EM, Hugosson J, Heijnsdijk EA, et al. To be screened or not to be screened? Modeling the consequences of PSA screening for the individual Br J Cancer 107778–7842012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent Eur Urol 71618–6292017 [DOI] [PubMed] [Google Scholar]
- 4.Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: A cost-effectiveness analysis Ann Intern Med 158853–8602013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial Eur Urol 63101–1072013 [DOI] [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer N Engl J Med 370932–9422014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishan AU, Shaikh T, Wang PC, et al. Clinical outcomes for patients with Gleason score 9-10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: A multi-institutional comparative analysis Eur Urol 71766–7732017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer J Urol 169517–5232003 [DOI] [PubMed] [Google Scholar]
- 9.Artibani W, Porcaro AB, De Marco V, et al. Management of biochemical recurrence after primary curative treatment for prostate cancer: A review Urol Int 100251–2622018 [DOI] [PubMed] [Google Scholar]
- 10.Pishgar F, Ebrahimi H, Saeedi Moghaddam SF, et al. Global, regional and national burden of prostate cancer, 1990 to 2015: Results from the global burden of disease study 2015 J Urol 1991224–12322018 [DOI] [PubMed] [Google Scholar]
- 11.Maurer T, Eiber M, Fanti S, et al. Imaging for prostate cancer recurrence Eur Urol Focus 2139–1502016 [DOI] [PubMed] [Google Scholar]
- 12.Turpin A, Girard E, Baillet C, et al. Imaging for metastasis in prostate cancer: A review of the literature. Front Oncol. 2020;10:15. doi: 10.3389/fonc.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy JAMA 2811591–15971999 [DOI] [PubMed] [Google Scholar]
- 14.Zagars GK, Pollack A.The fall and rise of prostate-specific antigen. Kinetics of serum prostate-specific antigen levels after radiation therapy for prostate cancer Cancer 72832–8421993 [DOI] [PubMed] [Google Scholar]
- 15.Spieler B, Goldstein J, Lawrence YR, et al. Salvage radiation therapy for biochemical failure following radical prostatectomy Isr Med Assoc J 1919–242017 [PubMed] [Google Scholar]