Abstract
PURPOSE
Poly (ADP-ribose) polymerase inhibitors (PARPi) have proven efficacy in treatment of BReast CAncer (BRCA) gene mutation-positive platinum-sensitive ovarian cancers. There is paucity of data for their role in platinum-resistant ovarian cancer (PROC). We report here retrospective analysis of outcome of PARPi treatment in a group of patients including those of PROC.
PATIENTS AND METHODS
We analyzed all consecutive patients who received PARPi. The efficacy of PARPi monotherapy was assessed in patients with relapsed high-grade serous ovarian carcinoma with gBRCAm. The drug was procured through compassionate program. Drugs (olaparib and talazoparib) were provided in capsule form.
RESULTS
Between July 1, 2015, and June 30, 2019, 28 patients with ovarian cancer received PARPi. At the time of data censoring (September 30, 2019), four (14.3%) patients are still on treatment. Median age was 54.5 years (range, 39-75 years). Median number of previous lines of chemotherapy received was three (range, 1-6). Eleven platinum-sensitive patients received the drug as maintenance (five in complete response and six in partial response after chemotherapy), whereas 17 (60.7%) had platinum-resistant progressive disease while starting the drug. In PROC, objective response rate (complete response plus partial response) was 47%, median progression-free survival was 8.2 months (5.3-11.3), and overall survival was 14.9 months (11.2-18.5). No new side effects were observed.
CONCLUSION
This is the first study from India evaluating PARPi in platinum-resistant ovarian cancer. This study suggests that PARPi is a viable treatment option in patients with PROC with gBRCAm. This should be further evaluated in randomized clinical trial.
INTRODUCTION
Epithelial ovarian cancer (EOC) is the third most common gynecologic cancer among women in the world and accounts for 6.6/100,000 new cases per year, whereas in India, it is second most common gynecologic cancer among women with an age-standardized rate of 4.9/100,000.1 The majority of patients with ovarian cancer relapse after a median progression-free survival (PFS) of 12-18 months.2-13 Outcome for patients with relapse is generally poor with median PFS from 6 to 12 months, which decreases with each relapse (8-12 months in platinum-sensitive cases and 3-6 months in platinum-resistant).14-18 Despite good response to initial chemotherapy, 75% patients die of their disease-related complications.19 Therefore, new approaches are needed to improve outcomes.
CONTEXT
Key Objective
What is the role of poly (ADP-ribose) polymerase inhibitors (PARPi) in relapsed platinum-resistant ovarian cancers (PROC) with germline gBRCA mutation?
Knowledge Generated
In PROC, a substantial number of patients (47%) respond to PARPi. The response lasts for a median of 8 months.
Relevance
In PROC with gBRCA1/2m, PARPi is an effective nonchemotherapeutic option.
The improved understanding of mechanisms of EOC has led to the identification of BReast CAncer gene mutation (BRCA) and homologous recombination deficiency (HRD) status as novel predictive biomarkers of response to chemotherapy as well as to poly (ADP-ribose) polymerase inhibitor (PARPi) treatment.20 To date, niraparib, olaparib, and rucaparib are the only PARPi that have been approved by the US Food and Drug Administration and European Medicines Agency in patients with EOC. It is approved in maintenance settings both in the first-line and subsequent lines for patients who achieved a complete response (CR) or partial response (PR) following chemotherapy. It is also approved in treatment of platinum-sensitive relapsed cases. Here, we report a retrospective analysis evaluating the role of PARPi in platinum-resistant ovarian cancer (PROC). The data have been presented as a poster in ESMO ASIA 2019 (abstr 237p).
PATIENTS AND METHODS
Patients
All patients with EOC who received PARPi between July 1, 2015, and June 30, 2019, at BL Kapur Memorial Hospital, New Delhi, were identified and data were extracted retrospectively. All the patients provided written informed consent. The drug was procured through compassionate program. Drugs were provided in capsule form. Patients were treated continuously with oral olaparib 400 mg twice a day (capsule formulation) or capsule talazoparib 1 mg once daily monotherapy until disease progression or drug discontinuation criteria were met. Hematologic and nonhematologic toxicities were assessed by Common Terminology Criteria for Adverse Events v4.0 (CTCAE) and dose modifications were done as per guidelines.
Data Collection
Patient demographics, tumor staging and pathologic data, treatment-related variables such as number of lines of chemotherapy received, platinum sensitivity, and follow-up data were retrieved. Ethics committee has approved this retrospective collection of data and study for publication.
Statistical Analysis
SPSS version 22 (IBM Corp, Armonk, NY) was used to analyze. The results are expressed as either median (range) or mean ± standard deviation. Comparisons between categorical variables were analyzed using the χ2 test. Continuous variables were expressed as medians and ranges and compared using the Mann-Whitney test. Variables influencing overall and progression-free survival rates were compared using the univariate and multivariate Cox regression analysis. Besides this, Kaplan-Meier method with logrank comparison was also used. The results are reported as a hazard ratio with 95% CIs. The P < .05 was considered statistically significant.
Outcome Measures and Definitions
We report the objective response rate (CR and PR in patients with measurable disease ie, CR plus PR), clinical benefit rate (CR, PR or stable disease for 24 weeks in patients with measurable disease ie, CR plus PR plus stable disease [as defined by RECIST v1.1]), duration of treatment, progression-free survival (PFS), overall survival (OS), and toxicity rate in patients with ovarian cancer (ovary, fallopian tube, and primary peritoneal). Efficacy data are stratified according to patients’ platinum sensitivity status. Platinum-sensitive disease is defined as achievement of a response (CR or PR) with recurrence or disease progression after 6 months of completion of the last dose of platinum-based chemotherapy. Resistant disease is defined when the time of recurrence from the last platinum treatment was < 6 months of completion of last dose of platinum-based chemotherapy.
PFS was calculated from the date of start of drug to the date of the first indication of disease progression or death, whichever occurred first; the data for patients who were alive without disease progression were censored as on the date of data censoring. OS was calculated from the date of start of drug to the date of death from any cause; data for patients still alive were censored at the date the patient was last known to be alive.
Duration of treatment was defined as the date from the start of drug to the date drug was last taken.
The data were censored on September 30, 2019.
RESULTS
Patients
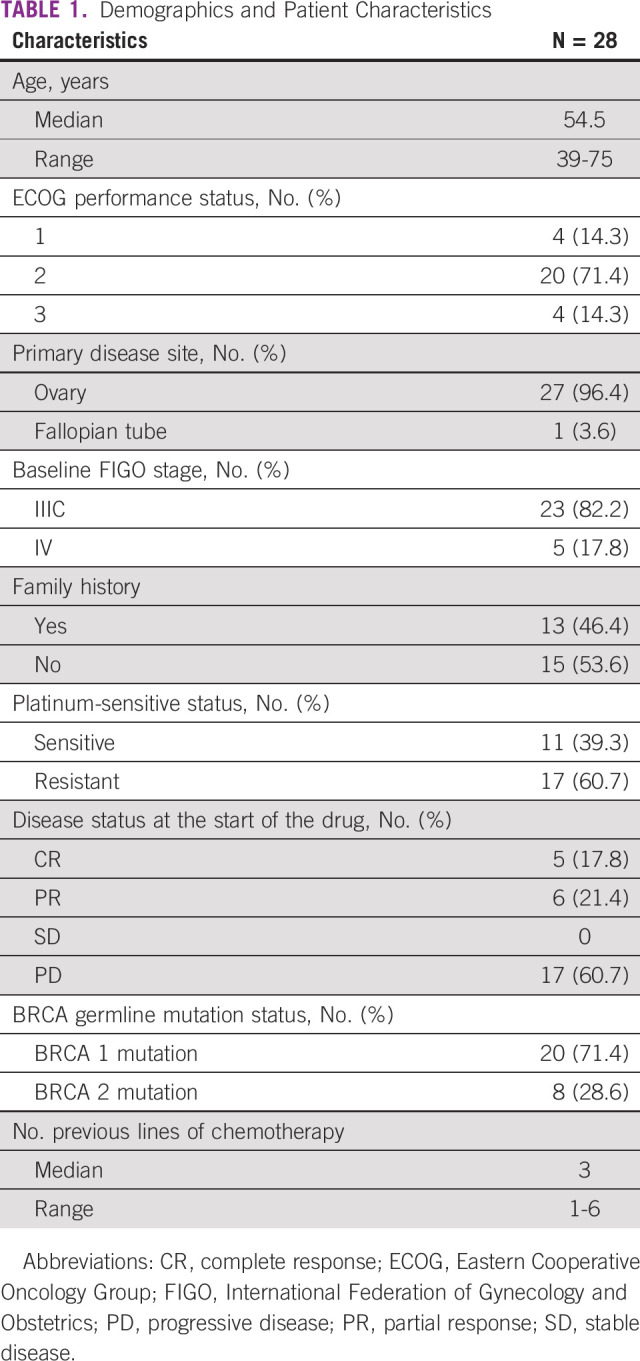
In the study period, 250 nonconsecutive patients with ovarian cancer were tested for gBRCAm, of which 40 (16%) were reported positive. Twenty-eight patients with EOC received PARPi. Eleven patients had platinum-sensitive and 17 had platinum-resistant disease at the time of starting PARPi. Five (17.8%) patients received talazoparib and 23 (82.2%) olaparib. Demographics and baseline patient characteristics have been described in Table 1. At the time of data censoring, four (14.3%) patients are still on treatment.
TABLE 1.
Demographics and Patient Characteristics
Outcome in Overall Patient Population
With the median treatment duration of 11.7 months (95% CI, 10.7 to 18.3), 78.57% patients eventually progressed. At the median follow-up of 15.4 months (range, 1.17 to 45.67 months), the median PFS was 10.14 months (95% CI, 5.9 to 17.5 months).
Outcomes in Platinum-Sensitive and Platinum-Resistant Subsets
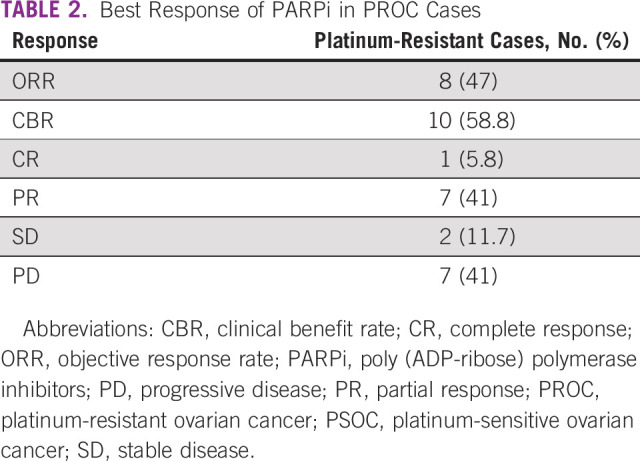
Among 11 patients with platinum-sensitive ovarian cancer (PSOC), five patients were in CR and six in PR while starting PARPi. One of the six patients who started PARPi in PR achieved a CR while on PARPi. The rest maintained their response on subsequent evaluation. The response of PARPi in platinum-resistant patient population is shown in Table 2.
TABLE 2.
Best Response of PARPi in PROC Cases
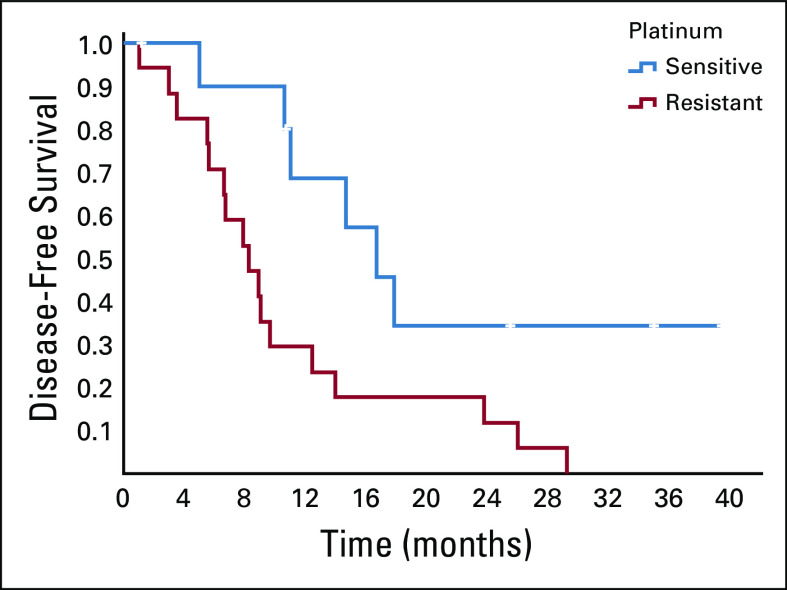
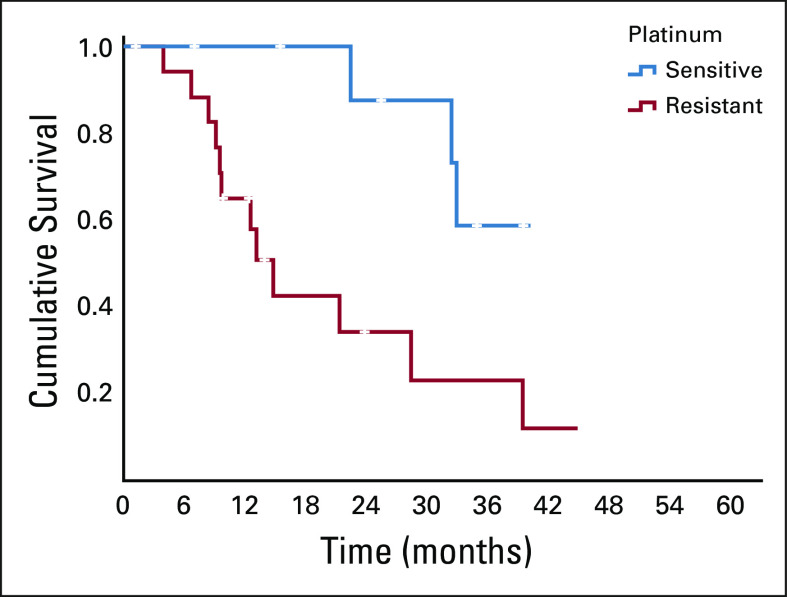
The patients with platinum-sensitive disease had significantly better PFS and OS as compared to the resistant subset (Table 3). Figures 1 and 2 show the Kaplan-Meier curve for PFS and OS of platinum-sensitive and platinum-resistant subsets. PSOC had trended toward better treatment duration, 16.9 months versus 9.2 months (P = .063).
TABLE 3.
Comparison of Median of Treatment Duration, PFS, and OS of PSOC and PROC Cases
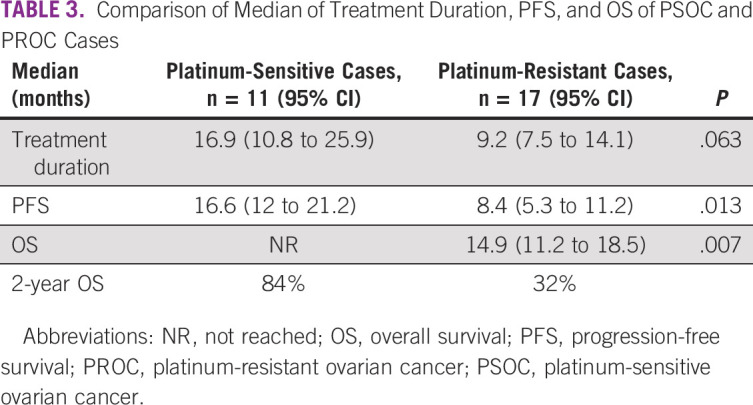
FIG 1.
PFS in PSOC and PROC cases. PFS, progression-free survival; PROC, platinum-resistant ovarian cancer; PSOC, platinum-sensitive ovarian cancer.
FIG 2.
OS in PSOC and PROC cases. OS, overall survival; PROC, platinum-resistant ovarian cancer; PSOC, platinum-sensitive ovarian cancer.
Most common side effects were fatigue (84%) and loss of appetite (72%). Grade ≥ 3 side effects were documented in 33% patients (anemia in 23.5%, thrombocytopenia in 17.6%, and fatigue 25%). All the patients with toxicity continued the drug with reduced doses except one. One patient developed myelodysplastic disease and another acute myeloid leukemia.
Median dose of olaparib received was 200 mg BD and talazoparib 1 mg OD.
DISCUSSION
There are few treatment options available for the patients with PROC. Three PARPi are approved in OC in the platinum-sensitive setting. Olaparib have been evaluated in PROC as well.21-24
In our study, objective response rate in platinum-resistant tumors was 47%, which was slightly higher as compared to that in Study 42 and CLIO study, where it was 30% and 36%, respectively.23,24
PFS ranged from 11.2 to 19 months in PSOC and 2.9 to 5.5 months in PROC in various studies.23-26 In our study, PFS was comparable in the platinum-sensitive subset, 16.9 months, whereas it was higher in the platinum-resistant patient population, 8.4 months. It may be because we did imaging and documented progression when patients became symptomatic instead of following strict 3 monthly evaluations as ovarian cancer has very limited treatment options once it becomes platinum-resistant. In some cases, we continued the PARPi if patient was clinically well and had controlled CA 125 and did not evaluate radiologically. This is in line with clinical practice in community.
Two-year OS in PSOC in this study was 82%, which is close to the reported 84% in the SOLO2 trial.26 None of the studies that evaluated PARPi in platinum-resistant recurrent setting has reported OS. In our study, patients with PROC had a median OS of 14.9 months (11.2-18.5) with a 2-year OS of 32%. In the studies that evaluated single-agent chemotherapy with or without bevacizumab in PROC, the 2-year OS ranged between 20% and 35%.27-29 This suggests that PARPi can be evaluated as a treatment option in gBRCAm cases. This needs to be proven in randomized trials.
Side effects were similar to that mentioned in literature including incidence of hematologic malignancy, which was 7% (two patients) in our study. The updated SOLO 2 data showed 8% incidence of hematologic malignancy in patients who received four or more lines of treatment.30,31 Both of our patients received PARPi after four or more lines of treatment.
Although the numbers of patients were very small, the results we report suggest that PARPi monotherapy has relatively high activity, even after multiple lines of chemotherapy, and is associated with objective responses in 47% of patients with clinically meaningful duration of response, and tolerable toxicity in patients with platinum-resistant ovarian cancer with gBRCAm. We did not do somatic BRCA mutation and HRD analysis. We cannot comment on whether these results can be extrapolated to the patients with HRD and somatic BRCA mutation.
In conclusion, PARPi monotherapy is a viable treatment option in patients with gBRCA1/2m ovarian cancer including platinum-resistant patients. The safety profile was consistent with that observed in previously reported PARPi monotherapy studies. To our knowledge, this is the first study from India evaluating it in platinum-resistant ovarian cancer. This should be further evaluated in randomized clinical trials.
ACKNOWLEDGMENT
Dr Prabhat Malik, Associate Professor, IRCH, AIIMS, New Delhi, India, helped in some part of data analysis.
Amit Aggarwal
Employment: Lilly
Stock and Other Ownership Interests: Lilly
No other potential conflicts of interest were reported.
DISCLAIMER
The views expressed in the submitted article are my own and not an official position of the institution or funder.
PRIOR PRESENTATION
Presented as poster in ESMO ASIA 2019 (abstr 237) on November 21, 2019, Singapore.
SUPPORT
Drug was provided by AstraZeneca under compassionate program.
AUTHOR CONTRIBUTIONS
Conception and design: Amit Agarwal, Saphalta Baghmar, Chandragouda Dodagoudar
Administrative support: Vikas Vaibhav
Provision of study materials or patients: Amit Agarwal, Saphalta Baghmar, Chandragouda Dodagoudar, Vikas Vaibhav
Collection and assembly of data: Amit Agarwal, Saphalta Baghmar, Chandragouda Dodagoudar, Suhail Qureshi, Vikas Vaibhav
Data analysis and interpretation: Amit Agarwal, Saphalta Baghmar, Chandragouda Dodagoudar, Suhail Qureshi, Aseem Khurana, Guresh Kumar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Amit Aggarwal
Employment: Lilly
Stock and Other Ownership Interests: Lilly
No other potential conflicts of interest were reported.
REFERENCES
- 1. www.uicc.org/new-global-cancer-data-globocan-2018
- 2.du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer J Natl Cancer Inst 951320–13292003 [DOI] [PubMed] [Google Scholar]
- 3.Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer J Clin Oncol 183084–30922000 [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group Study J Clin Oncol 213194–32002003 [DOI] [PubMed] [Google Scholar]
- 5.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase III trial of the Gynecologic Cancer Intergroup J Clin Oncol 271419–14252009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsumata N, Yasuda M, Isonishi S, et al. Long-term follow-up of a randomized trial comparing conventional paclitaxel and carboplatin with dose-dense weekly paclitaxel and carboplatin in women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: JGOG 3016 trial. J Clin Oncol. 2012;30 doi: 10.1016/S1470-2045(13)70363-2. 15 suppl; abstr 5003. [DOI] [PubMed] [Google Scholar]
- 7.Chan JK, Brady MF, Penson RT, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer N Engl J Med 374738–7482016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pignata S, Scambia G, Katsaros D, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial Lancet Oncol 15396–4052014 [DOI] [PubMed] [Google Scholar]
- 9.Clamp A, McNeish I, Dean A, et al. ICON 8: A GCIG phase III randomised trial evaluating weekly dose-dense chemotherapy integration in first-line epithelial ovarian/fallopian tube/primary peritoneal carcinoma (EOC) treatment: Results of primary progression-free survival (PFS) analysis. Ann Oncol. 2017;28 doi: 10.1016/S0140-6736(19)32259-7. suppl 5; abstr 929O_PR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer N Engl J Med 3652473–24832011 [DOI] [PubMed] [Google Scholar]
- 11.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer N Engl J 3652484–2496Med2011 [DOI] [PubMed] [Google Scholar]
- 12.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial Lancet Oncol 16928–9362015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randall L, Burger R, Nguyen H, et al. Outcome differences in patients with advanced epithelial ovarian, primary peritoneal and fallopian tube cancers treated with and without bevacizumab Gynecol Oncol 130e33–e342013 [Google Scholar]
- 14.Alberts D, Liu P, Wilczynski S, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200) Gynecol Oncol 10890–942008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aghajanian C, Blank S, Goff B, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer J Clin Oncol 302039–20452012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozas G, Bamias A, Koutsoukou V, et al. Biweekly gemcitabine and cisplatin in platinum-resistant/refractory, paclitaxel-pretreated, ovarian and peritoneal carcinoma Gynecol Oncol 104580–5852007 [DOI] [PubMed] [Google Scholar]
- 17.Bryant C, Kumar S, Spannuth W, et al. Feasibility of extension of platinum-free interval with weekly bolus topotecan and subsequent platinum retreatment outcomes in recurrent ovarian cancer Arch Gynecol Obstet 283361–3672011 [DOI] [PubMed] [Google Scholar]
- 18.Bombo N, Kutarska E, Dimopoulos M, et al. Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer J Clin Oncol 303841–38472012 [DOI] [PubMed] [Google Scholar]
- 19.Loizzi V, Chan JK, Osann K, et al. Survival outcomes in patients with recurrent ovarian cancer who were treated with chemoresistance assay-guided chemotherapy Am J Obstet Gynecol 1891301–13072003 [DOI] [PubMed] [Google Scholar]
- 20.Ganguly B, Dolfi SC, Rodriguez-Rodriguez L, et al. Role of biomarkers in the development of PARP inhibitors Biomark Cancer 815–252016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation J Clin Oncol 33244–2502015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matulonis UA, Penson RT, Domchek SM, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: A multistudy analysis of response rates and safety Ann Oncol 271013–10192016 [DOI] [PubMed] [Google Scholar]
- 23.Domchek SM, Aghajanian C.Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy Gynecol Oncl 140199–2032016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderstichele A, Nieuwenhuysen EV, Han S, et al. Randomized phase II CLIO study on olaparib monotherapy versus chemotherapy in platinum-resistant ovarian cancer. J Clin Oncol. 2019;37 suppl; abstr 5507. [Google Scholar]
- 25.Ledermann JA, Pujade-Lauraine E. Olaparib as maintenance treatment for patients with platinum-sensitive relapsed ovarian cancer. Ther Adv Med Oncol. 2019;11:1758835919849753. doi: 10.1177/1758835919849753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauraine EP, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial Lancet Oncol 181274–12842017 [DOI] [PubMed] [Google Scholar]
- 27.Martín AG, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer N Engl J Med 3812391–24022019 [DOI] [PubMed] [Google Scholar]
- 28.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial J Clin Oncol 321302–13082014 [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Rajappa S, Advani SH, et al. Prevalence of germlineBRCA1 and BRCA2 mutations and variants among ovarian, primary peritoneal and fallopian tube cancer patients: A multicentre Indian study Ann Oncol 30ix77–ix902019suppl 9 [Google Scholar]
- 30.Korach J, Turner S, Milenkova T, et al. Incidence of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients (pts) with a germline (g) BRCA mutation (m) and platinum-sensitive relapsed ovarian cancer (PSR OC) receiving maintenance olaparib in SOLO2: Impact of prior lines of platinum therapy. J Clin Oncol. 2018;36 suppl 15; abstr 5548. [Google Scholar]
- 31.Poveda A, Floquet A, Ledermann JA, et al. Final overall survival from SOLO2-ENGOT: A phase 3 trial assessing maintenance olaparib in patients with platinum sensitive relapsed ovarian cancer and a BRCA mutation. J Clin Oncol. 2020;38 suppl; abstr 6002. [Google Scholar]