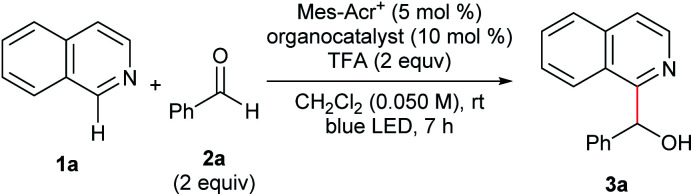
Optimization of the reaction conditionsa.
| ||
|---|---|---|
| Entry | Organocatalyst | 3a b (%) |
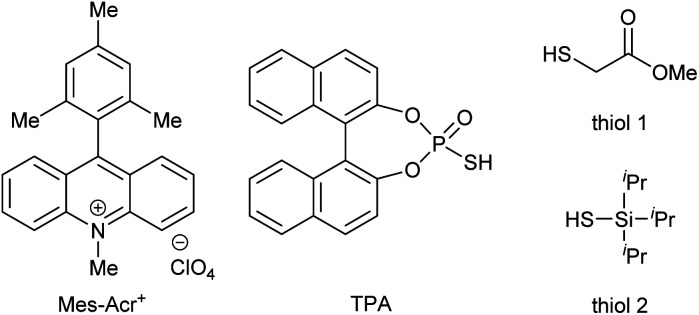
| 1 | TPA | 94 (89)c |
| 2 | Thiol 1 | ND |
| 3 | Thiol 2 | ND |
| 4 | Quinuclidine | 3 |
| 5 | Benzoic acid | ND |
| 6d | TPA | 29 |
| 7 | — | ND |
| 8e | TPA | 1 |
| 9f | TPA | ND |
| 10g | TPA | ND |
| ||
General reaction conditions: 1a (0.10 mmol), 2a (0.20 mmol), Mes-Acr+ (0.005 mmol), TPA (0.010 mmol), and TFA (0.20 mmol) were reacted in dichloromethane (DCM; 2.0 mL) at room temperature under blue LED irradiation for 7 h.
Yield was determined by 1H NMR analysis of the crude mixture using 1,1,2,2-tetrachloroethane as an internal standard.
Isolated yield in parentheses.
Without Mes-Acr+.
Without TFA.
Without photoirradiation.
1 equiv. TEMPO was added.
