Fig. 7. Model for the binding mechanism of the fuzzy α-actinin-2/FATZ-1 complex and potential implications in Z-disk ultrastructure and biogenesis.
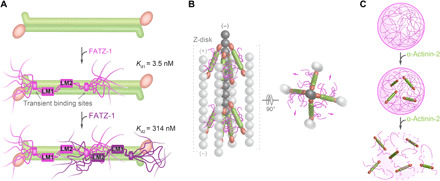
(A) Mechanism of binding between α-actinin-2 (in green) and FATZ-1, showing how the first FATZ-1 molecule (magenta) binds tightly to α-actinin-2 through both main molecular recognition elements (LM1 and LM2) and secondary binding sites, while the second FATZ-1 molecule (dark purple), which does not enjoy the same bonus of additional interactions as the first one, binds with lower affinity. (B) Model of the α-actinin-2/FATZ-1 complex [color code as in (A)] in the Z-disk, showing the polar architecture of the complex due to the binding of both FATZ-1 molecules to the conserved concave side of α-actinin-2. This might provide orientational constraints on α-actinin-2 rod and thus contribute to the paracrystalline tetragonal lattice of actin filaments (light and dark gray; actin dimers shown as a single ball for simplicity). (C) FATZ-1 phase-separates and forms biomolecular condensates with α-actinin-2 [color code as in (A)]. FATZ-1 condensates are prevented and dissolved by increasing concentrations of α-actinin-2, opening a potential novel avenue for sarcomere biogenesis starting from FATZ-1 condensates.
