Fig. 1. Nfia/b/x genes suppress proliferation and neurogenesis in tanycytes of neonatal mice.
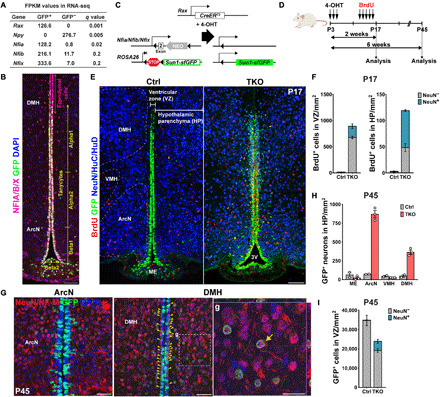
(A) Expression of Nfia/b/x in GFP+ tanycytes isolated from Rax:GFP mice (17) compared to the GFP− cells in adult hypothalamus. The tanycyte-specific marker Rax and the neuronal marker Npy are enriched in GFP+ and GFP− cells, respectively FPKM, fragments per kilobase of transcript per million mapped reads. (B) Distribution of Nfia/b/x protein in Rax-GFP+ tanycytes. Schematic of mouse lines is used in this study. (C and D) Schematic of a genetic approach for simultaneous tanycyte-specific disruption of Nfia/b/x and reporter gene labeling of tanycytes and tanycyte-derived cells using tamoxifen-dependent activation of CreER. (E) Induction of proliferation and neurogenesis in NFI-deficient tanycytes by P17. The Bregma position of the control section is slightly posterior to that of the mutant. 3V, the third ventricle. (F) Quantification of proliferation and neurogenesis in the VZ and hypothalamic parenchyma (HP) at P17 (n = 3 to 5 mice). (G) In NFI TKO mice by P45, mature neuronal marker NeuN and neurofilament M (NF-M) were simultaneously detected in TDNs migrating into the parenchyma of the arcuate nucleus (ArcN) and dorsomedial hypothalamus (DMH), with a small number of neurons remaining in the subventricular zone (yellow arrowheads). Enlarged image of parenchymal TDNs in (g) with the orthogonal view showing costaining within the cell. (H) Substantially increased numbers of NeuN+/GFP+ TDNs are observed in NFI TKO mice in ArcN and DMH relative to wild-type controls, but comparable numbers of neurons are observed in median eminence (ME) and ventromedial hypothalamus (VMH) (n = 2 to 3 mice). (I) The number of GFP+ tanycytes is reduced in NFI-deficient mice at P45, and ectopic neurons are seen in the VZ (n = 2 to 3 mice). Scale bars, 100 μm (B and E), 50 μm (G), and 25 μm (g).
