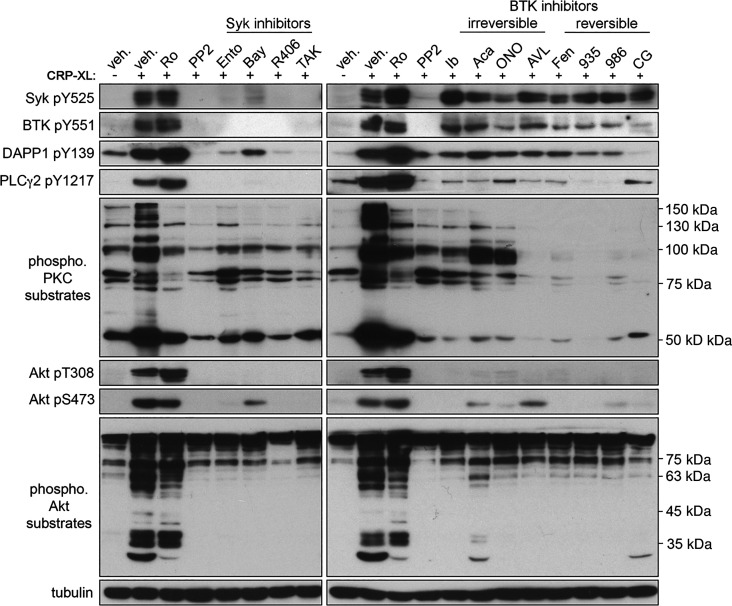
Figure 4.
Effects of Syk and BTK inhibitors on platelet activation signaling proteins. Replicate samples (n = 3) of purified, washed human platelets (1 × 109/mL) were incubated with the selected Syk and BTK inhibitors or with (0.1% DMSO) in solution and stimulated with CRP-XL. After collection into Laemmli sample buffer, platelet lysates were separated by SDS-PAGE and transferred to nitrocellulose. Western blots were conducted using antibodies for phosphorylated Syk Y525, BTK Y551, DAPP1 Y139, PLCγ2 Y1217, Akt T308, Akt S473, Akt substrates, and PKC substrates; α-tubulin serves as a loading control. Positions of molecular weight (kDa) markers relative to phosphorylated PKC and Akt substrates are indicated. Results representative of n = 4 experiments are shown. AVL, AVL-292 (spebrutinib); Bay, Bay 61-3606; BTK, Bruton’s tyrosine kinase; CG, CG-806; Fen, fenebrutinib; ONO, ONO-4059 (tirabrutinib); PLCγ2, phospholipase C γ2; R406, fostamatinib; Syk, spleen tyrosine kinase; veh., vehicle; 935, BMS-935177; 986, BMS-986195; Ento, entospletinib; Ib, ibrutinib; TAK, TAK-659; Ro, Ro 31-8220; CRP-XL, cross-linked collagen-related peptide; Aca, acalabrutinib.