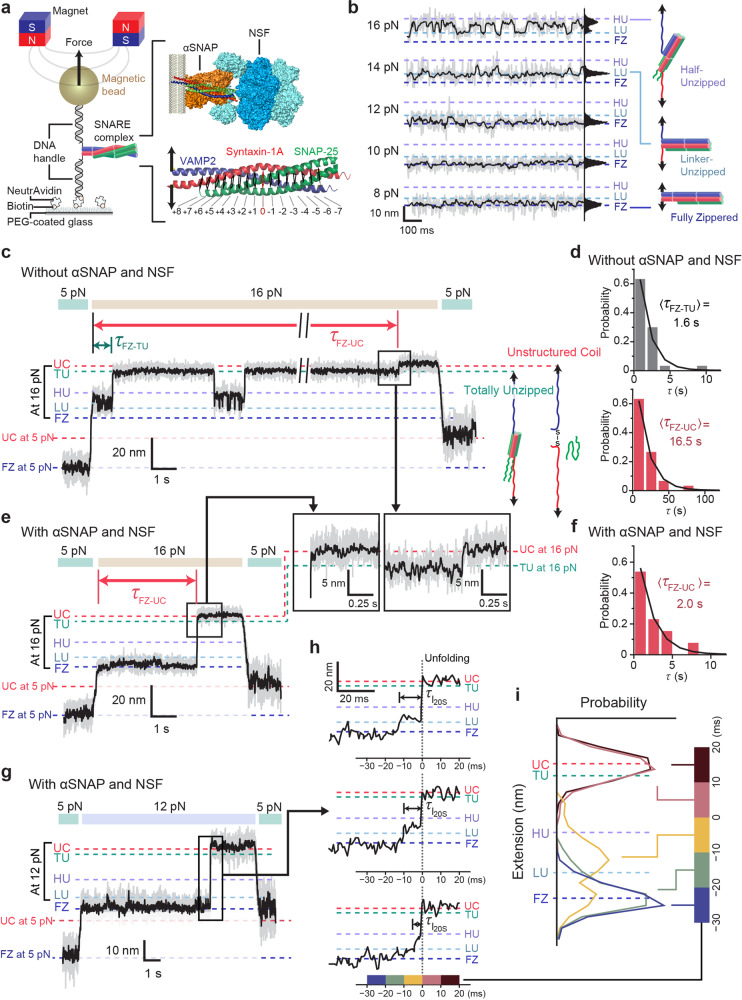
Fig. 1. NSF and αSNAP actively disassemble SNAREs via millisecond-long intermediates.
a Magnetic tweezers setup for observing SNARE complexes. A cartoon of membrane-bound 20S complex is shown for comparing the force-acting points. b Extension traces recorded at varying force levels with corresponding histograms. c, e Representative results from force-jump experiments without (c) and with (e) αSNAP and NSF (1 μM each). Both experiments were carried out with ATP (2 mM) and MgCl2 (10 mM). d, f Distributions of latency to unzipping (FZ to TU) and unfolding (FZ to UC) in force-jump experiments without (d) and with (f) αSNAP and NSF. g Force-jump experiment for 20S complex-mediated disassembly at 12 pN. The arrow indicates the region magnified in (h). h Representative high-speed traces of 20S complex-mediated disassembly. τI20S: intermediate state lifetime. Color code (bottom) represents the interval for the histograms shown in (i). i Extension distributions before and after the moment of disassembly collected from 26 disassembly events. The extension values were aligned with UC at zero for simplicity. Throughout the figure, the gray and black traces are 1.2-kHz raw and 60-Hz-filtered traces, respectively. FZ fully zippered, LU linker-unzipped, HU half-unzipped, TU totally unzipped, UC unstructured coil.