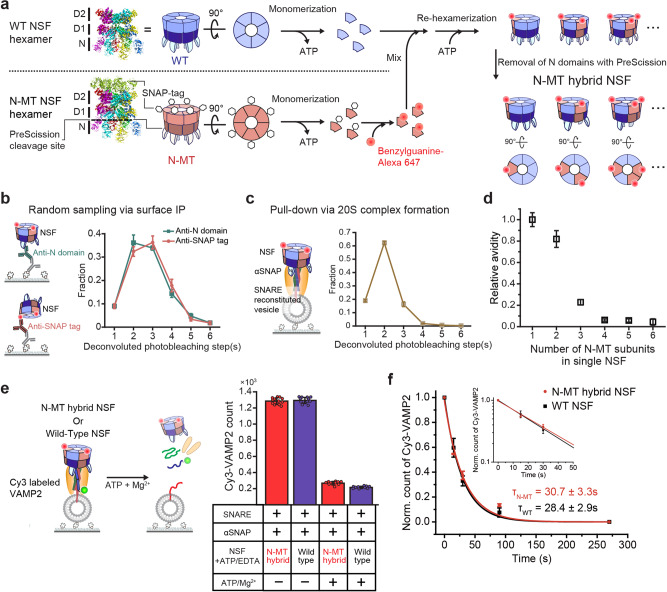
Fig. 3. Observation of cooperativity between NSF subunits in 20S complex formation and its disassembly function.
a Preparation of N-MT hybrid NSF including its monomerization, labeling, re-hexamerization, and N-domain removal (PDB ID: 3J9423 for NSF, 3KZZ for SNAP tag). b Experimental scheme of surface immunoprecipitation (IP) for the pulldown of N-MT hybrid NSF using two types of antibodies (N-domain and SNAP tag) and the distributions of deconvoluted photobleaching step(s) considering 90% labeling efficiency. c Experimental scheme of NSF pulldown via the SNARE–αSNAP complex and the distributions of deconvoluted photobleaching step(s). d Relative avidity of N-MT hybrid hexamers to the SNARE–αSNAP complex according to the number of N-MT subunits in single NSF hexamers. Data in (c) divided by those using the anti-N-domain antibody in (b) and normalized to the value for single-mutant hexamers. e Measurements of WT and N-MT hybrid NSF’s disassembly activity. Counts for SNARE complexes under ATP-non-hydrolyzing (1 mM ATP/1 mM EDTA) and inducing ATP hydrolysis (1 mM ATP/10 mM Mg2+) for 5 min. f Normalized counts of Cy3-VAMP2 under ATP-hydrolyzing condition with two types of NSF at several time points. Inset shows a semi-log plot of early time points. τ represent the time constant of exponential decay. The data represent mean ± s.e.m. for four independent experiments (b, c). The data in (d) represent the normalized values obtained by dividing the data in (c) by the data using the anti-N-domain antibody in (b) ± error propagated by the calculations. The data represents mean ± s.d. for n = 12 (-ATP/Mg2+) or 10 (+ATP/Mg2+) images from two independent experiments (e) and mean ± s.e.m. for three independent experiments (f). Source data are provided as a Source Data file.