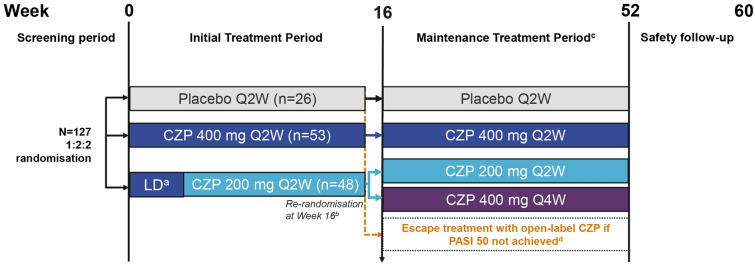
Fig. 1.
Study design. aPatients received a loading dose of CZP 400 mg at weeks 0, 2 and 4; bpatients initially randomised to CZP 200 mg Q2W were re-randomised (1:1) to receive either CZP 200 mg Q2W or CZP 400 mg Q4W (with placebo administered on alternate dosing weeks to maintain the blind); cpatients who achieved PASI 50 continued therapy to week 52; dpatients who did not achieve PASI 50 entered an open-label escape arm and received CZP 200 mg Q2W (CZP 400 mg Q2W loading dose for the first three visits). CZP certolizumab pegol, LD loading dose, PASI 50 ≥ 50% reduction from baseline in Psoriasis Area and Severity Index, Q2W every 2 weeks, Q4W every 4 weeks