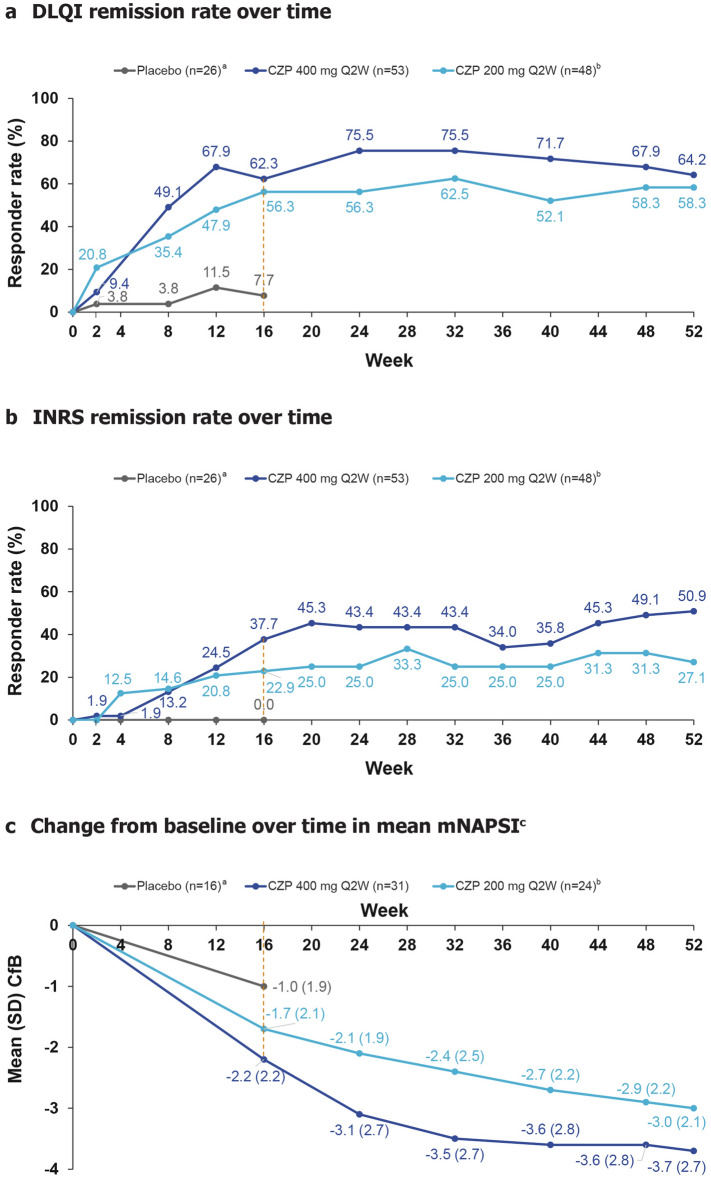
Fig. 5.
Other efficacy outcomes to week 52. Responder rates and mean change from baseline are summarised according to patients’ originally randomised treatment group. Missing values were imputed using NRI (for DLQI and INRS) and LOCF for mNAPSI. aOnly four patients continued placebo treatment in maintenance; therefore, placebo data are only shown to week 16; bincludes patients who received CZP 200 mg Q2W and CZP 400 mg Q4W during maintenance; cmNAPSI was assessed only in patients with psoriatic nail disease at baseline. CfB change from baseline, CZP certolizumab pegol, DLQI Dermatology Life Quality Index, INRS Itch Numeric Rating Scale, mNAPSI modified Nail Psoriasis Severity Index, NRI non-responder imputation, Q2W every 2 weeks, Q4W every 4 weeks, SD standard deviation