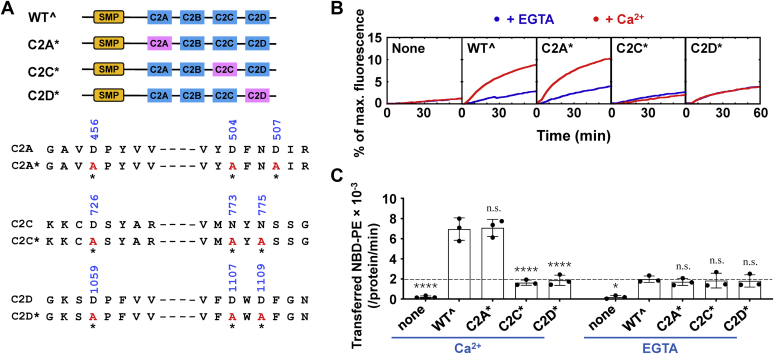
Figure 4.
The Ca2+-dependent lipid transfer activity of Tcb3 requires the intact Ca2+-binding sites on both C2C and C2D domains.A, Top, schematic diagrams of WTˆ and mutant Tcb3 proteins. WTˆ C2 domains are shown in blue, and mutant C2 domains are shown in pink. Bottom, sequences showing the aspartic acid or asparagine residues in the C2A, C2C, and C2D domains of Tcb3 that coordinate Ca2+ binding. Mutated residues are indicated with residue numbers shown on the top and asterisks shown at the bottom. B, lipid transfer of the protein-free liposomes in the absence or presence of 1 μM Tcb3 (WTˆ or mutants). The lipid transfer reactions included 0.1 mM EGTA or CaCl2. C, initial lipid transfer rates of NBD-labeled phosphatidylethanolamine in the reactions shown in B. The dashed line indicates the level of Ca2+-independent lipid transfer. Error bars indicate standard deviation. Data are presented as mean ± SD (n = 3 independent replicates). p Values were calculated using two-way ANOVA with Tukey’s multiple comparisons test. n.s., p > 0.05. ∗p = 0.0355. ∗∗∗∗p < 0.0001. F (4, 20) = 59.14, p < 0.0001 for protein factor and F (1, 20) = 90.03, p < 0.0001 for Ca2+ factor. A significant interaction between the two factors is F (4, 20) = 35.18, p < 0.0001.