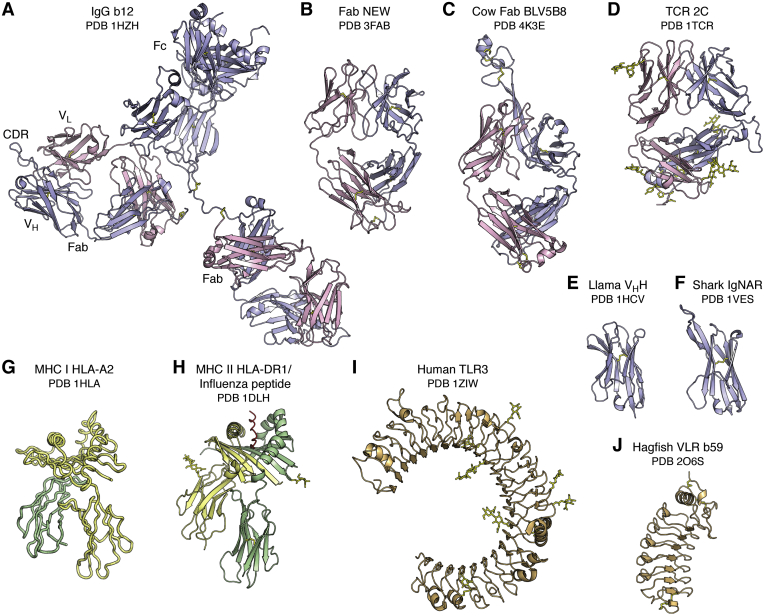
Figure 1.
The diversity of antigen receptors in the immune system. The immunoglobulin fold is utilized as the recognition motif in antibodies (A, B, C, E and F) in the humoral adaptive immune system and T cell receptors (D) in the cellular adaptive immune system. The intact IgG b12 (A) is labeled to illustrate the relative positions of the two Fab and one Fc regions, the VL and VH immunoglobulin domains within one Fab, and the complementarity-determining region containing region of the Fab. The camelid family (E), which includes llamas, and the shark family (F), also have smaller antibodies that contain only a single VH domain (termed VHH or nanobody) instead of the two VH and VL domains in conventional antibodies. The major histocompatibility complex (MHC) fold (G and H) is used to present peptide antigens to T cell receptors for classical MHC I and II, and lipids, glycolipids, specialized peptides, and other antigens in nonclassical MHC-like molecules. Note that, in (G), the unrefined HLA-A2 structure was deposited with only Cα atoms, so a cartoon trace is shown. Toll-like receptors (I) in the innate immune system adopt a Leu-rich repeat fold and recognize specific antigens, including proteins, nucleic acids, lipopolysaccharides, unmethylated CpG, and small molecules. Variable lymphocyte receptors (J) (VLRs) also are composed of Leu-rich repeats and function as the adaptive immune responses in the jawless vertebrates, lampreys, and hagfish. In this and following figures, the immunoglobulin light and heavy chains are colored pink and light blue. The MHC Class I heavy chain is colored yellow and β2 microglobulin chain in green, whereas the MHC Class II α and β chains are colored yellow and green. The TLRs and VLRs are colored in beige. For all figures, carbohydrate and disulfide bonds are colored yellow. The name of the receptor, ligand if any, and Protein Data Bank (PDB) ID are shown below each figure.