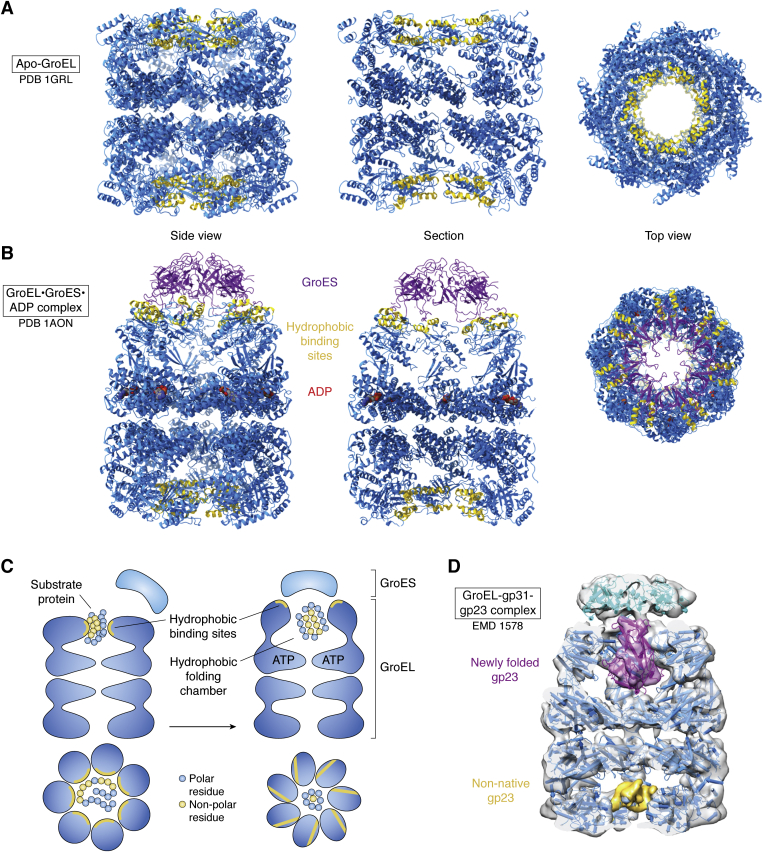
Figure 2.
GroEL-GroES and interactions with a nonnative substrate protein.A, the structure of apo GroEL, shown from the side, in a cut-away view and from the top ((10), 1grl). Helices H and I, which harbor many of the key hydrophobic substrate-binding sites (11), are shown in yellow. B, the equivalent representations of the GroEL-GroES-ADP complex, with GroES in purple and the nucleotide in red ((14), 1aon). C, cartoons showing GroEL side and top views with a partly unfolded protein captured on the hydrophobic binding sites (yellow) and then encapsulated and folded in the hydrophilic folding chamber of GroEL-GroES. D, section of a cryo EM map of GroEL bound to gp31, the bacteriophage T4 homologue of GroES, with a partly folded subunit of the T4 capsid protein gp23 (pink) inside the folding chamber, and partial density for a nonnative gp23 (yellow) in the open ring ((32), EMD-1548).