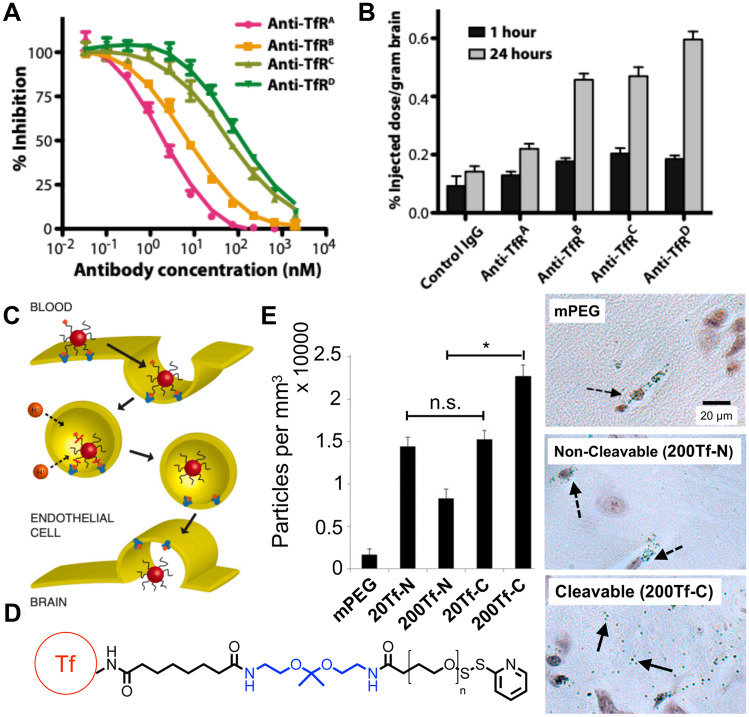
Fig. 3.
Strategies to reduce binding affinity with transferrin receptors on BBB endothelial cells increased delivery to the brain. A Yu et al. engineered a series of anti-TfR antibodies with varying TfR binding affinities. B Decreasing TfR binding affinity increased drug delivery to the brain especially at 24 h after IV administration of nanoparticles. Reproduced with permission from Yu et al. [73]. C Proposed mechanism for BBB transcytosis of NPs conjugated to holo-transferrin (holo-Tf) ligand via an acid-labile linkage. D Chemical structure of the acid-labile linker used by Clark and Davis to conjugate holo-Tf to NPs; holo-Tf in red, acid-labile linkage in blue. E NPs decorated with holo-Tf via acid cleavable bonds (Tf-C) induced significantly higher levels of NP accumulation in brain parenchyma compared to NPs modified with non-cleavable bonds (Tf-N) at high modification densities (*P < 0.0001). Histology images showed that NPs modified with Tf-N or mPEG were mostly entrapped in blood vessels, but NPs modified with Tf-C dispersed within brain parenchyma (dashed arrows indicate NPs within blood vessels; solid arrows indicate NPs in brain parenchyma). Reproduced with permission from Clark and Davis [75]