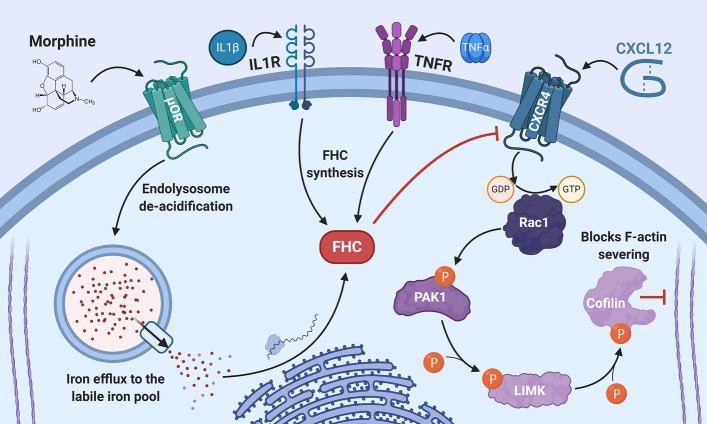
Fig. 3.
Opioid and inflammatory regulation of ferritin heavy chain and corresponding effects on CXCR4 signaling and dendritic spines. Our studies indicate that µ-opioid agonists and inflammatory signaling pathways in cortical neurons converge to upregulate ferritin heavy chain (FHC), an iron storage protein that also inhibits CXCR4 signaling and produces corresponding dendritic spine deficits. Morphine activation of µ-opioid receptors (µOR) promotes de-acidification of endolysosomes and release of endolysosomal iron. This increases free labile iron in the cytosol, to which neurons respond by post-transcriptionally upregulating FHC protein levels. In the same system, FHC is also upregulated by inflammatory signaling pathways, including downstream of IL-1β/IL1 receptor (IL1R) and TNFα/TNF receptor (TNFR) signaling. In each case, FHC inhibits activation of CXCR4 via its natural ligand CXCL12, which prevents CXCR4-induced stabilization of dendritic spines. Normally, CXCL12/CXCR4 signaling enhances dendritic spine density by increasing the amount of activated, GTP-bound Rac1, which promotes a cascading phosphorylation of PAK1, LIMK, and the actin severing protein cofilin. Phosphorylation of cofilin blocks its ability to break down synaptic actin, which stabilizes dendritic spines and also reverses spine deficits in HIV-transgenic rats