Fig. 1.
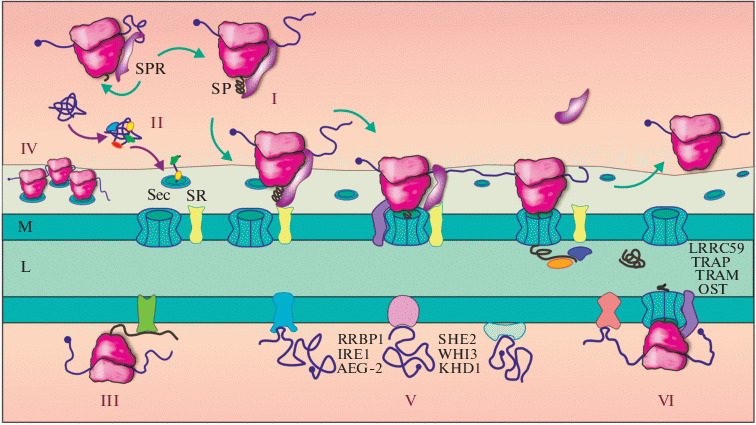
Diversity of mechanisms for protein targeting to the ER and localization of mRNA on the ER membrane. (I) The classic SRP-dependent pathway: the SRP particle, pre-associated with the ribosome, recognizes the signal peptide (SP) or transmembrane domain (TMD) emerging from the ribosomal tunnel, and arrests elongation, after which the transport of the mRNA/ribosome/nascent peptide/SRP complex onto the ER membrane (M) occurs, where SRP binds to its membrane receptor, SR. The translocon (Sec-complex) is involved in the binding of the ribosome on the ER and the translocation of the peptide across the membrane into lumen (L), with the help of proteins associated with the translocon (TRAP, TRAM, OST, etc.). (II) Post-translational mechanism of protein import into the ER mediated by chaperones and other proteins. (III) Localization through the interaction of the synthesized product with the ER resident protein. (IV) Retention of mRNA on the ER membrane in complex with the polysome. (V) Translation-independent mRNA localization mediated by 3'-untranslated region (3'-UTR). Some of the known mRNA-binding proteins involved in various mRNA localization pathways are shown. (VI) Association of the translation complex with the ER through membrane receptors of the ribosome and mRNA-binding proteins.
