Abstract
Objective
Migraine, endometriosis, and the comorbidity of both are frequent pain disorders of special relevance for women. The neuropeptide calcitonin gene‐related peptide (CGRP) is critically involved in migraine, and circumstantial evidence suggests a role in endometriosis. We assessed CGRP levels at different times of menstrual cycle in four groups: healthy women, women with migraine or endometriosis and with the comorbidity of both.
Methods
Women with episodic migraine and women with a histologically confirmed endometriosis were recruited from specialized centers. For CGRP determination with a commercial enzyme immunoassay kit, cubital vein blood samples were collected on menstrual cycle day 2 ± 2 (during menstruation) and on day 15 ± 2 (periovulatory period). The primary endpoint of the study was the absolute difference of CGRP plasma levels between the menstrual and the periovulatory phase of all study groups. Groups were compared using nonparametric test procedures.
Results
A total of 124 women were included in the study. The change of CGRP plasma levels between menstruation and the periovulatory period was different between groups (p = 0.007). Women with comorbid migraine and endometriosis showed an increase of CGRP in the menstrual phase of +6.32 (interquartile range, IQR −3.64–13.60) compared to the periovulatory time, while healthy controls had a decrease of −10.14 (−22.54–0.91, p = 0.004). CGRP levels were different in the periovulatory phase among groups (p = 0.008), with highest values in healthy controls.
Interpretation
CGRP levels change significantly during the menstrual cycle. Different patterns in women with the comorbidity point to a deviant regulation of CGRP release.
Introduction
Women's health remains a significant challenge across the globe. Endometriosis and migraine are both listed among the most prevalent diseases in women and share pain as their most predominant clinical feature. Endometriosis is a chronic inflammatory gynecological disorder affecting over 10% of women worldwide with a significant therapeutic need and so is migraine. 1 , 2 Each of them leads to an impairment of quality of life and exhibits detrimental effects on the physical and mental health in affected women. 2 , 3
Epidemiological studies report a significant comorbidity of migraine and endometriosis, and the presence of one of the two disorders also increases the chance to suffer from the other. 4 , 5 In migraine and in endometriosis, severe pain attacks occur often during the menstrual period. 1 , 2 Impaired regulation of inflammatory signaling pathways and neurotransmitter release, such as calcitonin gene‐related peptide (CGRP), may contribute to the development of acute pain episodes. 6 , 7
Acute migraine is clearly linked to CGRP release from trigeminal afferent neurons. 6 Hormonal fluctuations contribute to the generation of attacks, 8 but CGRP levels in women have yet to be determined during the menstrual cycle.
The increased density of CGRP‐positive sensory nerve fibers in affected tissues indicates a role of this neuropeptide in endometriosis. 7 Along with the proliferation and growth of endometriosis cells, CGRP seems to promote neurogenic inflammation in this tissue. 9 Despite these findings, CGRP‐ and CGRP‐related mechanisms have not been studied to date in vivo in women with endometriosis.
We therefore studied CGRP levels in women during the menstrual cycle at two predefined timepoints, that is, during menstruation and in the periovulatory period (PO). It was our hypothesis that CGRP levels increase during menstruation in parallel to the perimenstrual (PM) estrogen drop with a difference between women with the comorbidity of migraine and endometriosis, women with migraine and women with endometriosis. We assumed that women with the comorbidity of migraine and endometriosis have most pronounced CGRP level changes based on the assumption that both conditions are CGRP‐related.
Methods
Study design and participants
This was a single‐center, longitudinal, observational cohort trial conducted at the Headache Center, Department of Neurology, Charité—Universitätsmedizin Berlin, Germany. The approved Endometriosis Center, Department of Gynecology, Charité and an outpatient Endometriosis Center (ADE) recruited patients with endometriosis. Healthy female controls were recruited from hospital staff not related to the study team and medical students through direct approach or announcements in mailing lists.
The study consisted of four study groups: women with episodic migraine, women with endometriosis, women with endometriosis and migraine, and female healthy controls.
For inclusion, all females had to have a regular menstrual cycle, defined as menstrual cycle duration between 25 and 35 days in the 3 months prior to screening.
Patients with migraine had to fulfill the criteria of episodic migraine according to the International Classification of Headache Disorders 3rd Edition (ICHD‐3) 10 and to have had at least one migraine attack in the 4 weeks prior to screening. The use of migraine prophylactic medications was not allowed.
For enrollment into one of the endometriosis study groups, a histologically confirmed diagnosis of endometriosis was required, in addition to existing pelvic pain (self‐classified as endometriosis‐related) at least once in the four weeks prior to screening.
The exclusion criteria have been: use of hormonal contraception, treatment with sex hormones or sex hormone modulators, any other suspected or present gynecological or neurological disease, and diagnosis of chronic migraine or any other diagnosed primary headache disorder except tension‐type headache on less than 2 days in the month prior to screening.
For healthy controls, further exclusion criteria applied: Females were excluded if they had a history of primary headache apart from tension‐type headache on less than 2 days per month and/or if they reported strong pelvic pain or cramps during menstruation.
The Charité Ethical Committee (EA1/165/18) approved the study. All participants gave written informed consent following study information. The study was registered in the German Clinical Trial Register (DRKS00020744).
Study procedures
The study consisted of three on‐site visits, lasting approximately 1 hour each: a screening visit, a visit during menstruation at day 2 ± 2 (perimenstrual visit = PM), and a visit in the intermenstrual period at day 15 ± 2 of the menstrual cycle (periovulatory visits = PO). The first day of menstrual bleeding is defined as day 1 of the menstrual cycle.
The patient's medical history with a focus on migraine and/or endometriosis‐related symptoms and treatment was taken at screening, followed by a physical examination. A headache‐experienced neurologist confirmed the diagnosis of migraine in both migraine groups and classified all reported headache attacks in the month prior to screening as migraine or tension‐type headache, in order to assess the inclusion and exclusion criteria. For the grading of endometriosis severity we used the revised classifications of the American Society for Reproductive Medicine (rASRM), 11 as documented in the patient's gynecologic record: stage I corresponds to a minimal severity, stage II mild, stage III moderate, and stage IV severe.
At screening, patients with migraine completed the Headache Impact Test‐6 (HIT‐6) 12 and patients with endometriosis the Endometriosis Health Profile‐30 (EHP‐30). 13 The HIT‐6 is a standardized, validated questionnaire to assess negative effects of headache on daily life 12 and comprises of six questions. Each question is answered on a 5‐point scale. The total global score ranges from 36 to 78, and a score >60 indicates a severe impact of headache on quality of life.
The EHP‐30 is a validated, 30‐item questionnaire assessing the health‐related quality of life in patients with endometriosis. 13 The items are divided in five dimensions: pain (11 items), control and powerlessness (6), social support (4), emotional well‐being (6), and self‐image (3). For each dimension, a percentage score (0–100) is built. Lower scores indicate a better health status.
At study visits PM and PO, participants reported the first day of their current menstrual cycle, the date of their last migraine attack and/or endometriosis‐related pain episode, and the last intake of acute pain medication. Blood samples for the analysis of CGRP were drawn between 8–12 AM on each visit from the antecubital vein but only when patients were free of any migraine symptoms since more than 12 hours and at least 12 hours after the last intake of any acute pain medication.
We collected the blood samples in precooled 10 ml EDTA tubes (BD Vacutainer®), prepared with 500 µl aprotinin (5–10 trypsin inhibitor unit (TIU)/ml) (Sigma Aldrich, Munich, Germany). Immediately afterwards, the tubes were centrifuged at −6°C and 2000 rpm, for 15 minutes. Plasma was transferred into 1.5 ml polypropylene tubes (Eppendorf, Hamburg, Germany) and stored at −80°C. CGRP concentrations were measured via a commercial enzyme immunoassay‐KIT (EIA) (Bertin Bioreagent, Montigny le Bretonneux, France), following manufacturer's instructions. This two‐site immunometric assay combines an anti‐N terminus antibody with an anti‐C terminus antibody and is equally sensitive for all human CGRP isoforms. 14 The limit of detection is 2 pg/ml. 14 All samples of one patient were analyzed in the same kit, and each kit contained samples of all four study groups in similar proportions. Two samples from the first kit were measured in each of the following kits and served as additional quality control and reference standard.
We aimed to schedule PM and PO within the same menstrual cycle, but if not possible, the missing visit was scheduled up to 2 months later.
Outcomes
The primary endpoint of the study was the absolute difference of CGRP concentrations in pg/ml from the menstrual to the PO period (i.e., PM – PO) between all study groups. Secondary endpoints were the relative difference in CGRP concentrations [100 – (PO*100/PM)] as well as the absolute CGRP concentrations at each of the two time points.
Statistical analysis
Based on a previous study of CGRP levels in patients with chronic migraine, 15 we assumed a large effect size of d = 0.9 for the primary endpoint. A sample size of 25 patients per group was therefore sufficient to detect an effect of similar magnitude with a statistical power of 0.85 at a significance level of α = 0.05 (two‐tailed) using the Kruskal–Wallis analysis of variance (ANOVA). Assuming a dropout rate of 15%, we planned to enroll at least 30 patients per group. The power analysis was conducted with G*Power. 16
Data at screening were summarized with descriptive statistics, using means ± standard error for numerical variables and frequencies and percentages for categorical variables. Only participants who completed study protocol were included in the analysis of primary and secondary endpoints. We tested the primary and secondary outcome measures for normal distribution using the Shapiro–Wilks test. Because data were not normally distributed, we compared outcomes between groups using the Kruskal–Wallis (ANOVA) with post hoc Mann–Whitney U tests. Within‐group analyses were performed using the Wilcoxon test. Due to the influence of older age and obesity on CGRP release, 17 , 18 we examined the effects of age and BMI on the primary outcome usingsemi‐parametric probabilistic index models. 19 Correlations between CGRP concentrations and migraine and endometriosis features were explored using Kendall's nonparametric correlation coefficient τ. Kendall's τ can assume values between −1 and 1: The closer the value comes to 0, the weaker the correlation. 20
All statistical analyses were performed using R (version 3.6.2; The R Foundation for Statistical Computing, Vienna, Austria. Primary and secondary outcomes are reported as median and interquartile range (IQR). In line with common recommendations, p values are reported only for the primary and secondary endpoints. The significance level was corrected for multiple comparisons in post hoc tests using Bonferroni's correction.
Results
Between 8 January 2019 and 18 November 2019, we screened 134 individuals and enrolled 122 females in the study (n = 31 in group MM, n = 30 in group EE, n = 30 in group ME, and n = 31 in group HC). The study was completed by 110 participants (90.2%); reasons for noncompletion are shown in Figure 1. All study visits were performed during the same menstrual cycle in 89 patients (80.9%).
Figure 1.
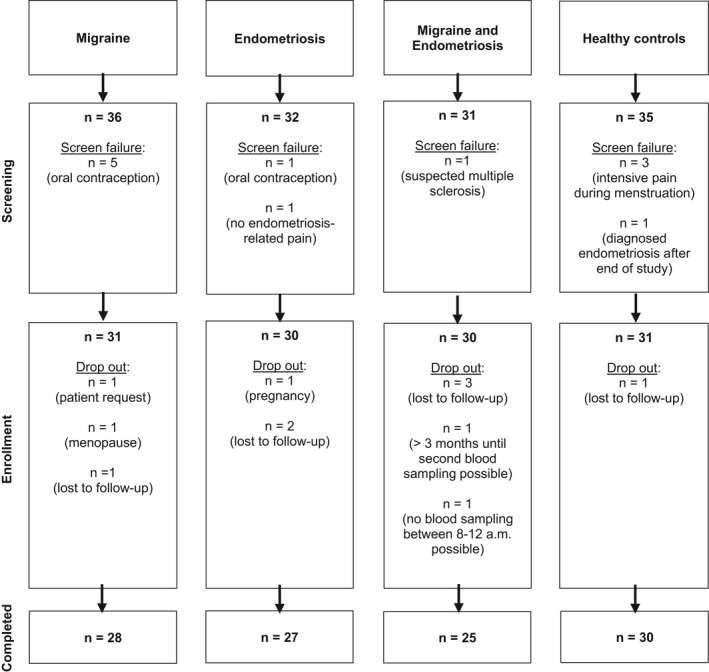
Flowchart of participant selection.
All groups were similar in age and BMI (Table 1). Migraine characteristics were similar between the two migraine groups. Patients with migraine only treated their attacks more frequently with triptans than comorbid patients, who had higher use of nonsteroidal anti‐inflammatory drugs (NSAIDs). Patients with migraine and endometriosis patients reported a higher number of days with endometriosis‐related pain, pain intensity, and attack duration than patients with endometriosis only. However, rASRM disease scores were similar between the two groups.
Table 1.
Demographic, anamnestic, and clinical features of study participants.
| Patient characteristics | ||||
|---|---|---|---|---|
| Healthy controls | Migraine | Endometriosis | Migraine and endometriosis | |
| Age | 31.55 ± 1.71 | 32.74 ± 1.31 | 31.90 ± 1.02 | 35.70 ± 1.32 |
| BMI | 22.76 ± 3.09 | 24.11 ± 3.73 | 24.29 ± 4.13 | 23.76 ± 4.22 |
| Migraine characteristics | Endometriosis characteristics | ||||
|---|---|---|---|---|---|
| Migraine | Migraine and endometriosis | Endometriosis | Migraine and endometriosis | ||
| Years since migraine diagnosis | 16.16 ± 1.63 | 17.13 ± 2.06 | Years since endometriosis diagnosis | 3.03 ± 0.66 | 4.90 ± 1.09 |
| Monthly migraine days | 5.48 ± 0.57 | 4.53 ± 0.75 | Monthly days with endometriosis pain | 4.27 ± 0.77 | 5.48 ± 0.87 |
| Pain intensity | 7.19 ± 0.22 | 7.00 ± 0.29 | Pain intensity | 5.77 ± 0.46 | 6.43 ± 0.44 |
| Attack duration (h) | 28.92 ± 3.94 | 29.13 ± 4.94 | Attack duration (h) | 17.50 ± 3.27 | 26.77 ± 4.36 |
| Aura | 5 (17.9%) | 2 (8.0%) | rASRM score | 2.13 ± 0.22 | 2.08 ± 0.29 |
| Positive family history | 20 (71.4%) | 16 (64.0%) | Positive family history | 9 (33.3%) | 4 (16.0%) |
All values are reported as means (±standard error) or n (%).
Abbreviations: BMI, body mass index; NSAID, nonsteroidal anti‐inflammatory drugs; rASRM, revised classifications of the American Society for Reproductive Medicine.
Patients in both migraine groups reported a severe impact of headache on their quality of life with HIT‐6 scores of 63.45 ± 0.90 (migraine only) and 60.73 ± 1.19 (migraine and endometriosis). The EHP‐30 scores were higher in almost every domain in comorbid patients than in patients with endometriosis only, indicating a stronger impact of endometriosis symptoms in patients with migraine and endometriosis on their perceived health status (Figure 2). The difference was particularly evident in the domain “Pain” with an average score of 35.99 ± 4.42 (endometriosis only) and 53.28 ± 3.76 (migraine and endometriosis). This shows a greater influence of endometriosis‐related pain on quality of life in comorbid women.
Figure 2.
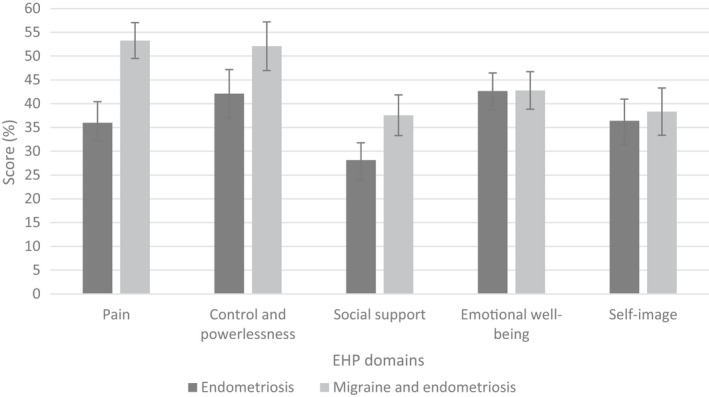
Endometriosis Health Profile‐30 (EHP‐30) scores (mean ± standard error) in five domains in women with endometriosis (dark gray bars) and women with migraine and endometriosis (light gray bars). Higher scores indicate a worse perceived endometriosis‐related health status in the respective domain.
Changes in plasma CGRP levels during the menstrual cycle
The absolute difference in CGRP levels from the PM to the PO was different between all groups (p = 0.007). Statistical analyses revealed that comorbid patients had an increase of +6.32 pg/ml (IQR −3.64 to 13.60) during menstruation compared to the PO, while healthy female controls showed a decrease of −10.14 (IQR −22.54 to 0.91, p = 0.004), as shown in Table 2. The relative change of CGRP concentrations from PM to PO was also significantly different between comorbid patients and healthy controls (12.5%, IQR −8.6 to 31.0 in comorbid women vs. −11.3%, IQR −29.9 to 1.6 in healthy controls, p = 0.022). Adjusting for the covariates age and BMI did not affect the results (p = 0.001 in the adjusted model).
Table 2.
Absolute and relative changes in CGRP plasma concentrations from PM to PO in the four analyzed study groups (median and IQR).
|
Absolute difference (pg/ml) PM ‐ PO |
Relative difference (%) PM ‐ PO |
|
|---|---|---|
| Migraine |
−2.44 IQR −18.37 to −2.45 |
−3.3 IQR −31.4 to 17.5 |
| Endometriosis |
−1.24 IQR −12.46 to 9.22 |
−3.4 IQR −29.2 to 17.8 |
| Migraine and endometriosis |
6.32 IQR −3.64 to 13.60 |
12.5 IQR −8.6 to 31.0 |
| Healthy controls |
−10.14 IQR −22.54 to 0.91 |
−11.3 IQR −29.9 to 1.6 |
| P value (all groups) | 0.007* | 0.037* |
| P value (pairwise) |
M – E: >0.999 M – ME: 0.245 E – ME: >0.999 M – HC: >0.999 E – HC: 0.198 ME – HC: 0.004* |
M – E: >0.999 M – ME: 0.549 E – ME: 0.676 M – HC: >0.999 E – HC: >0.999 ME – HC: 0.022* |
Abbreviations: CGRP, calcitonin gene‐related peptide; E, endometriosis; HC; healthy controls; IQR, interquartile range; M, migraine; ME, migraine and endometriosis; PM, perimenstrual; PO, periovulatory.
Statistically significant.
Absolute plasma CGRP levels during the menstrual cycle
All groups showed similar plasma CGRP concentrations during menstruation (PM) with 47.70 pg/ml (IQR 33.67–73.31) in patients with migraine, 46.35 pg/ml (IQR 32.48–64.11) in patients with endometriosis, 52.59 pg/ml (IQR 35.08–72.41) in comorbid patients and 55.01 pg/ml (IQR 42.78–130.08) in healthy controls (p = 0.324 across all groups).
In the PO, CGRP concentrations were significantly different between all groups (p = 0.011). Post hoc analyses revealed that healthy female controls had higher CGRP levels (67.34 pg/ml, IQR 49.60–134.06) when compared to women with migraine and endometriosis (46.21 pg/ml, IQR 34.10–59.56, p = 0.016). Women with migraine (47.39 pg/ml, IQR 34.45–104.33) and women with endometriosis (47.85 pg/ml, IQR 33.96–69.99) did not differ significantly from the other groups.
The analyses within groups showed that women with migraine (p = 0.210) or endometriosis (p = 0.962) had similar CGRP concentrations in PM and PO measurements. Comorbid women showed numerically higher CGRP concentrations at PM, but without statistical significance (p = 0.078). Healthy controls had higher concentrations at PO (p = 0.003).
Figure 3 illustrates CGRP concentrations at PM and PO for each group.
Figure 3.
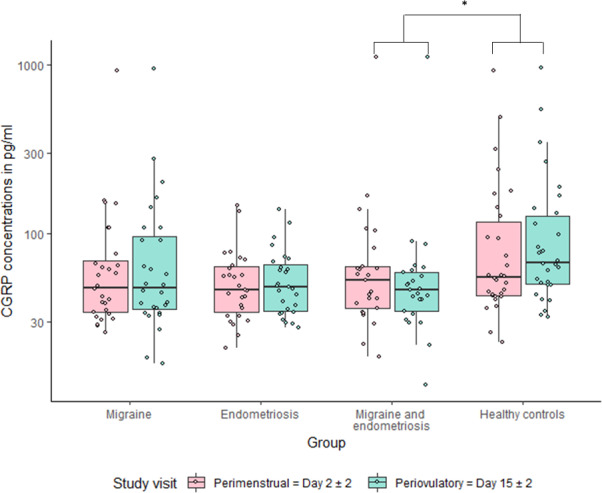
Boxplot and jitter plot for calcitonin gene‐related peptide (CGRP) plasma concentrations at the perimenstrual (PM) and periovulatory (PO) visit for each study group. The boxes are drawn from the 25% to 75% quartiles, the horizontal line represents the median. The whiskers are drawn out to 1.5*interquartile range. The dots represent single data points. a p = 0.078 between PM and PO. *p = 0.003 between PM and PO. **p = 0.004 for the changes from PM and PO between groups.
Data analysis did not reveal any correlation between CGRP levels and migraine headache or endometriosis pain frequency. The time interval since the last migraine or endometriosis‐related pain attack or the last intake of pain medication also showed no correlation to CGRP levels (Table 3).
Table 3.
Kendall's τ correlation coefficient between CGRP levels and time since the last migraine or endometriosis‐related pain attacks or the last intake of acute pain medication.
| Perimenstrual CGRP concentrations (PM) | Periovulatory CGRP concentrations (PO) | |
|---|---|---|
| Migraine frequency | −0.07 | −0.10 |
| Endometriosis frequency | 0.00 | −0.06 |
| Days since last migraine attack | 0.03 | 0.06 |
| Days since intake of acute migraine medication | 0.00 | 0.08 |
| Days since last endometriosis‐related pain | 0.04 | −0.05 |
| Days since last intake of acute pain medication (endometriosis) | −0.17 | 0.05 |
A value < 0.2 or > −0.2 indicates a weak correlation.
Abbreviations: CGRP, calcitonin gene‐related peptide; PM, perimenstrual; PO, periovulatory.
Discussion
This study shows that CGRP levels change during the menstrual cycle. In women with the comorbidity of migraine and endometriosis CGRP levels rise during menstruation. In contrast, healthy females display a decrease of CGRP during the menstrual period, while changes in women with only migraine and women with only endometriosis do not reach statistical significance. PO CGRP concentrations are lower in comorbid women than in healthy controls. The fluctuation of CGRP levels during the female cycle in healthy women is consistent with a possible influence of female sex hormones on CGRP release. The diversion of this pattern in women with migraine and endometriosis points to disease‐specific pathophysiological events, which may be explained by a different regulation of CGRP release in females with this comorbidity.
The multitude of factors that influence CGRP homeostasis include ovarian sex hormones. 21 In 1986, Stevenson et al. observed increased CGRP plasma levels during pregnancy, which returned to normal after delivery. 22 Women of reproductive age displayed higher CGRP plasma levels than men. 23 These studies suggest a direct relationship between estrogen levels and CGRP concentrations in blood, which is in line with our observations in healthy females.
In contrast, patients with the comorbidity of migraine and endometriosis display an increase of CGRP in parallel to the perimenstrual estrogen drop. The cause might be found in genetically determined differences underlying this comorbidity, as shared molecular genetic mechanism has been recently proposed by Adewuyi et al. 24 In fact, with TRIM32 and SLC35G6, two loci have been identified in a genome wide association study to be associated with this comorbidity. These loci are enriched for mitogen‐activated protein kinase (MAPK) and TNF‐a signaling, which may lead to a different hormone‐dependent CGRP release than in healthy women. 24
It is unlikely that high menstrual CGRP concentrations in comorbid females are merely a consequence of acute migraine or endometriosis pain. The study visits occurred after a similar time interval from the last acute pain attack in all patient groups, and analyses did not find a correlation between CGRP concentrations and the number of days from the last pain attack.
This study shows that CGRP plasma levels do not differ in the menstrual period and at a later time point in the menstrual cycle between women with migraine and healthy female controls. Previous research showed mixed results on plasma CGRP levels in patients with episodic migraine. 15 , 25 , 26 , 27 Our results may also differ from these observations as previous studies included mixed‐sex cohorts and did not consider the influence of the menstrual cycle and hormonal therapies.
We expected higher CGRP plasma levels in women with endometriosis based on intense CGRP staining in endometriotic lesions, 9 which may translate into enhanced CGRP release into the blood. While similar CGRP concentrations in women with endometriosis and healthy controls are unexpected, they do not exclude a role of CGRP in the pathophysiology of endometriosis. Numerous inflammatory signaling pathways are involved in the pathogenesis of endometriosis. 2 The interactions between the individual pathways and regulatory mechanisms are highly complex and were not within the scope of this study. Moreover, CGRP may only accumulate near endometriosis lesions and dilution in cubital vein blood does not allow the detection of local activity changes and fluctuations.
Absolute CGRP plasma concentrations in the literature vary based on EIA or radioimmunoassay (RIA) laboratory methodology. Mean CGRP values range from ~10–140 pg/ml for healthy controls to ~20–250 pg/ml for migraine patients with large deviations in each case. 28 , 29 Our median values of ~50 pg/ml fit well into this range. Some outliers were detected in the control group, in which four women had values >200 pg/ml at both time points, leading to higher median values. The reason for these observations remains to be determined. All women in the control group had been carefully selected and had no pre‐existing conditions and normal vitals, minimizing the risk of systematic errors.
Migraine and endometriosis are relevant women's health issues. 2 , 3 The disease burden increases when both condition occur together, and comorbid patients suffer more often from depression and anxiety. 30 In line with this observation, the EHP‐30 scores in our study revealed a stronger impact of endometriosis on the perceived health status in comorbid patients. In particular, endometriosis‐related pain had a more negative effect on patients' life.
The careful selection of women with the confirmed histological diagnosis of endometriosis and the migraine diagnosis according to ICHD‐3 criteria by experienced headache specialists represent a strength of this study. CGRP determination in cubital vein blood can be affected by dilution. 31 Nevertheless, this is a standard method to determine neuropeptide levels in headache disorders. Based on the evidence in the literature, we focused this research on CGRP as the most obvious common underlying molecule of migraine and endometriosis. Other possible targets of interest (e.g., Substance P or Prostaglandin E2) are lacking of strong evidence in one or the other disease and were therefore not within the scope of this investigation. For example, Substance P antagonists are not effective in the therapy of migraine. 32
All samples were collected under standardized conditions across all groups. The pre‐analytical processing with the addition of a protease inhibitor in the vials, immediate centrifugation, and consistent maintenance of the cooling chain minimized CGRP degradation.
In conclusion, our data provide first evidence for a cycle‐dependent CGRP release in healthy women and in women with the comorbidity of migraine and endometriosis albeit in a different pattern. Women with only migraine or endometriosis showed similar CGRP concentrations across the menstrual cycle. The findings support the hypothesis of a distinct pathophysiological background in comorbid patients probably due to a specific underlying genetic profile.
Author Contributions
BR and UR designed the study. BR, LHO, JM, MDH, HK, and LN contributed to collection of data. ADE, JS, and SM contributed to the recruitment of study participants. BR, LHO, CPN, and UR analyzed the data. BR and UR wrote the first draft of the manuscript. All authors were involved in the interpretation of the data. All authors critically reviewed and edited the manuscript.
Conflicts of Interest
BR reports grants from Novartis, during the conduct of the study, personal fees from Novartis, Teva, and Allergan, all outside the submitted work. LHO has nothing to disclose. JM reports personal fees from Novartis, outside the submitted work. MDH has nothing to disclose. HK has nothing to disclose. CPN has nothing to disclose. LN reports personal fees from Novartis, Allergan, TEVA, BIAL, Hormosan, and Eli Lilly, all outside the submitted work. ADE has nothing to disclose. JS reports personal fees from Roche, grants and personal fees from Eli Lilly, personal fees from Johnson and Johnson, all outside the submitted work. SM has nothing to disclose. UR reports personal fees from Abbvie, Allergan, Amgen, Eli Lilly, Medscape, Novartis, StreaMedUp, and Teva; institutional fees from Amgen, Eli Lilly, Novartis, Teva, and Alder, and grants from Novartis, all outside the submitted work.
Acknowledgments
We thank Sonja Blumenau for her technical assistance. The study was funded by a personal grant to BR from Novartis Pharma GmbH Germany. BR is a fellow in the BIH Charité Clinician Scientist Program funded by the Charité—Universitätsmedizin Berlin and the Berlin Institute of Health. The Program was initiated and lead by Professor Duska Dragun to enable resident physicians to pursue a career in academic medicine. Professor Dragun passed away on 28 December 2020. This publication is dedicated to her memory as a mentor, role model, and scientist. Open Access funding enabled and organized by Projekt DEAL
Funding Statement
This work was funded by Novartis Pharma GmbH grant .
References
- 1. Bulun SE. Endometriosis. N Eng J Med 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 2. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin 2019;37(4):631–649. [DOI] [PubMed] [Google Scholar]
- 3. Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27(5):1292–1299. [DOI] [PubMed] [Google Scholar]
- 4. Ferrero S, Pretta S, Bertoldi S, et al. Increased frequency of migraine among women with endometriosis. Hum Reprod 2004;19(12):2927–2932. [DOI] [PubMed] [Google Scholar]
- 5. Tietjen GE, Conway A, Utley C, et al. Migraine is associated with menorrhagia and endometriosis. Headache 2006;46(3):422–428. [DOI] [PubMed] [Google Scholar]
- 6. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28(2):183–187. [DOI] [PubMed] [Google Scholar]
- 7. Gupta D, Hull ML, Fraser I, et al. Endometrial biomarkers for the non‐invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;4(4):CD012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin VT, Lipton RB. Epidemiology and biology of menstrual migraine. Headache 2008;48(Suppl 3):S124–S130. [DOI] [PubMed] [Google Scholar]
- 9. Yan D, Liu X, Guo SW. Neuropeptides substance P and calcitonin gene related peptide accelerate the development and fibrogenesis of endometriosis. Sci Rep 2019;9(1):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 11. American Society for Reproductive . Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 12. Yang M, Rendas‐Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT‐6™) across episodic and chronic migraine. Cephalalgia 2011;31(3):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP‐30. Qual Life Res 2004;13(3):705–713. [DOI] [PubMed] [Google Scholar]
- 14. Frobert Y, Nevers MC, Amadesi S, et al. A sensitive sandwich enzyme immunoassay for calcitonin gene‐related peptide (CGRP): characterization and application. Peptides 1999;20(2):275–284. [DOI] [PubMed] [Google Scholar]
- 15. Cernuda‐Morollón E, Larrosa D, Ramón C, et al. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013;81(14):1191–1196. [DOI] [PubMed] [Google Scholar]
- 16. Faul F, Erdfelder E, Lang A‐G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 17. Tummanapalli SS, Willcox MDP, Issar T, et al. The effect of age, gender and body mass index on tear film neuromediators and corneal nerves. Curr Eye Res 2020;45(4):411–418. [DOI] [PubMed] [Google Scholar]
- 18. Marics B, Peitl B, Varga A, et al. Diet‐induced obesity alters dural CGRP release and potentiates TRPA1‐mediated trigeminovascular responses. Cephalalgia 2017;37(6):581–591. [DOI] [PubMed] [Google Scholar]
- 19. Thas O, Neve JD, Clement L, Ottoy J‐P. Probabilistic index models. J R Stat Soc Series B Stat Methodol 2012;74(4):623–671. [Google Scholar]
- 20. Abdi AH. The Kendall Rank Correlation Coefficient. 1 In: Salkind N (Ed), Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage, 2007. [Google Scholar]
- 21. Labastida‐Ramírez A, Rubio‐Beltrán E, Villalón CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia 2019;39(3):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevenson JCMD, Warren RC, et al. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed) 1986;293(6558):1329–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene‐related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest 1990;50(4):385–388. [DOI] [PubMed] [Google Scholar]
- 24. Adewuyi EO, Sapkota Y, Auta A, et al. Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes 2020;11(3):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashina M, Bendtsen L, Jensen R, et al. Evidence for increased plasma levels of calcitonin gene‐related peptide in migraine outside of attacks. Pain 2000;86(1–2):133–138. [DOI] [PubMed] [Google Scholar]
- 26. Gupta R, Ahmed T, Banerjee B, Bhatia M. Plasma calcitonin gene‐related peptide concentration is comparable to control group among migraineurs and tension type headache subjects during inter‐ictal period. J Headache Pain 2009;10(3):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia 1995;15(5):384–390. [DOI] [PubMed] [Google Scholar]
- 28. Fusayasu E, Kowa H, Takeshima T, et al. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache‐free periods. Pain 2007;128(3):209–214. [DOI] [PubMed] [Google Scholar]
- 29. Jang MU, Park JW, Kho HS, et al. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis 2011;17(2):187–193. [DOI] [PubMed] [Google Scholar]
- 30. Tietjen GE, Bushnell CD, Herial NA, et al. Endometriosis is associated with prevalence of comorbid conditions in migraine. Headache 2007;47(7):1069–1078. [DOI] [PubMed] [Google Scholar]
- 31. Lee MJ, Lee SY, Cho S, et al. Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain 2018;19(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diener HC. RPR100893, a substance‐P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia. 2003;23(3):183–185. [DOI] [PubMed] [Google Scholar]


