Abstract
Patient: Male, 21-year-old
Final Diagnosis: Antiphospholipid antibody syndrome • cerebral venous sinus thrombosis
Symptoms: Blurring of vision • confusion • fever • headache
Medication: —
Clinical Procedure: Laboratory • magnetic resonance imaging
Specialty: Neurology • Ophthalmology
Objective:
Rare disease
Background:
Cerebral venous sinus thrombosis (CVST) is a serious life- and vision-threatening condition that can have a variable presentation according to the site of venous occlusion, including mimicking idiopathic intracranial hyper-tension. We report on a patient with primary antiphospholipid antibody syndrome (APS) who presented with papilledema due to CVST that was refractory to medical treatment but responded to optic nerve sheath fenestration (ONSF).
Case Report:
A 21-year-old man presented with blurred vision of gradual onset and a progressive course for 1 month, accompanied by fever, headache, and confusion. He had a history of lower-limb deep vein thrombosis. Examination revealed decreased vision with bilateral grade IV papilledema. Magnetic resonance venography showed evidence of CVST and laboratory investigations revealed lupus anticoagulant antibodies, antinuclear antibodies, and anti-double stranded DNA antibodies, with hyperhomocysteinemia. The patient did not meet the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus (SLE) nor the new European League Against Rheumatism and American College of Rheumatology SLE classification criteria. He was diagnosed with CVST secondary to APS and hyperhomocysteinemia and treated with acetazolamide, systemic anticoagulation, and vitamins for 1 month, but with no improvement in his ophthalmic condition. He subsequently underwent unilateral ONSF, which resulted in improvement in vision bilaterally that continued throughout a 6-month follow-up period.
Conclusions:
Papilledema associated with CVST can be the first presentation of APS. When performed in a timely manner, ONSF can save useful vision and lead to improvement in vision in patients with papilledema due to CVST that is refractory to medical treatment.
Keywords: Antibodies, Antiphospholipid; Hyperhomocysteinemia; Intracranial Thrombosis; Lupus Vasculitis, Central Nervous System; Magnetic Resonance Imaging; Papilledema
Background
Cerebral venous sinus thrombosis (CVST) is a rare but life- and vision-threatening condition characterized by a clinical presentation that varies depending on the site of venous occlusion, including a resemblance to idiopathic intracranial hypertension (IIH) [1]. CVST manifestations include headaches, blurred vision, seizures, neurological deficits, and impaired consciousness [1]. Risks factors for it include coagulation disorders such as antiphospholipid antibody syndrome (APS) and hyperhomocysteinemia [2,3] and systemic inflammatory diseases such as systemic lupus erythematosus (SLE) and Behcet disease [4].
Standard treatment for CVST includes anticoagulation and therapy for the underlying cause, while acetazolamide is used to treat the associated papilledema and prevent optic atrophy and blindness [5,6]. Intravascular thrombolysis using chemical thrombolysis, mechanical thrombectomy, or a combination of both can be used in patients whose condition is deteriorating despite adequate anticoagulation [5]. These invasive measures, however, carry the risk of bleeding. Other new treatment modalities include cerebral venous sinus stenting, which also can lead to bleeding and death [7].
APS is characterized by development of arterial or venous thrombosis in the presence of lupus anticoagulant (LA), anti-cardiolipin antibody, and/or anti-β2-glycoprotein I; the specificity for thrombosis is highest for LA [8]. APS is classified as primary if it is not associated with a systemic autoimmune disorder and secondary if it is associated with one such as SLE [8].
We report on a patient with papilledema due to CVST associated with APS and hyperhomocysteinemia whose condition was refractory to systemic medical treatment but responded well to unilateral optic nerve sheath fenestration (ONSF). To the best of our knowledge, ours is the first report of such a case.
Case Report
A 21-year-old man was referred by an Internal Medicine specialist because of gradually progressive diminution of vision in both eyes over a 1-month period. He also reported having fever, confusion, and headache, which had started days before the diminution of vision. He had a history of deep vein thrombosis (DVT) in his left leg several years earlier, which was attributed to varicose veins and treated at that time with anticoagulation. He had no past ophthalmic history. Treatment at the time of referral included ibuprofen.
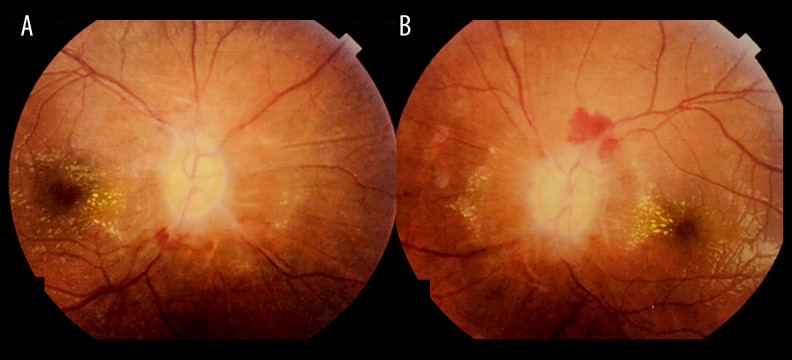
Examination of the patient revealed a corrected distance visual acuity (CDVA) of 20/400 in both eyes. Color vision testing was 11/20 in the right eye and 10/20 in the left using Ishihara plates. Pupillary examination did not reveal a relative afferent pupillary defect; however, pupillary light reflexes were sluggish. External ocular exam and ocular motility were normal. Anterior segment examination was unremarkable in both eyes. Posterior segment examination revealed bilateral grade IV papilledema (Frisén scale) with disc pallor and flame-shaped hemorrhages along the disc margins with hard exudates involving the macular area (Figure 1A, 1B).
Figure 1.
Posterior segment examination of the A: right and B: left eyes revealed bilateral grade IV papilledema with optic disc pallor and flame-shaped retinal hemorrhages along the disc margins, with hard exudates extending into the macular area.
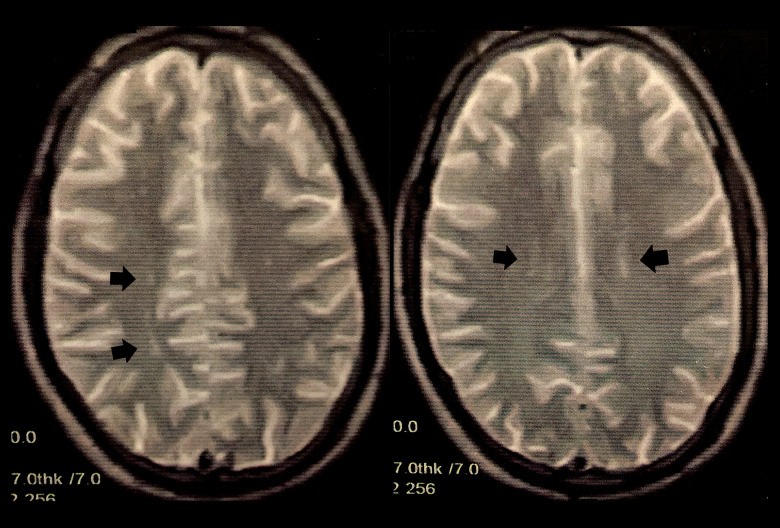
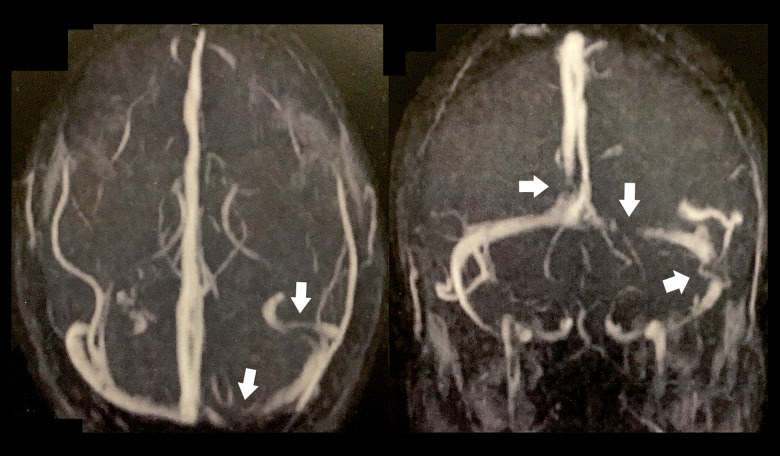
A review of systems was unremarkable and the patient’s rheumatological, neurological, and dermatological examinations were normal. He had a body mass index of 22.5. He was referred for magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) of the brain. MRI revealed a few bilateral, periventricular, oval-shaped foci of abnormal signal intensity scattered in the deep white matter, which were inconspicuous on T1-weighted imaging but with bright signals on T2-weighted images (Figure 2). Their longitudinal axis was perpendicular to the lateral ventricles, which was suggestive of multiple sclerosis. MRV showed multiple filling defects involving the proximal aspect of the superior sagittal sinus and the medial and lateral aspects of the left transverse sinus (Figure 3). Therefore, the patient was diagnosed with CVST and started on low-molecular-weight heparin, warfarin (5 mg/d), and acetazolamide tablets (1 g/d) with a target international normalized ratio (INR) of 2 to 3. He was also instructed to undergo extensive laboratory investigations, which revealed a normal serum creatinine level of 0.8 mg/dL (normal range, 0.5–1.5 mg/dL), normal urinalysis, a normal complete blood picture with no hematological abnormalities, prolonged partial thromboplastin time-lupus anticoagulant (PTT-LA) test (Diagnostica Stago, Parsippany, New Jersey, United States) at 48.6 s (normal range, 31–43 s) indicating positive lupus anticoagulant antibodies [9], normal anti-cardiolipin and anti-β2-glycoprotein I antibodies, elevated antinuclear antibodies at 30 IU/mL (normal range, <25 IU/ mL), elevated anti-double stranded DNA (anti-dsDNA) antibodies at 78 IU/mL (normal range, <25 IU/mL), elevated homocysteine levels at 47 µmol/L (normal range, 5–15 µmol/L), and elevated erythrocyte sedimentation rate at 33 mm after 2 h (normal range, ≤15 mm) (Table 1).
Figure 2.
Magnetic resonance imaging of the brain revealed a few bilateral periventricular, oval-shaped foci of abnormal signal intensity scattered in the deep white matter, with bright signals on T2-weighted images (black arrows).
Figure 3.
Magnetic resonance venography revealed multiple filling defects involving the proximal aspect of the superior sagittal sinus and the medial and lateral aspects of the left transverse sinus (white arrows).
Table 1.
Summary of the results of the laboratory investigations of the patient.
| Laboratory test | Value | Reference |
|---|---|---|
| Serum creatinine, mg/dl | 0.8 | 0.5–1.5 |
| Urine analysis | Normal | Normal |
| Complete blood picture | Normal | Normal |
| PTT-LA, seconds | 48.6* | 31–43 |
| Anti-cardiolipin antibodies | Negative | Negative |
| Anti-β2-glycoprotein I antibodies | Negative | Negative |
| Antinuclear antibodies, IU/mL | 30* | <25 |
| Anti-dsDNA antibodies, IU/mL | 78* | <25 |
| Erythrocyte sedimentation rate, mm | 33* (2 hours) | 15 (2 hours) |
Indicates abnormal test result. Anti-dsDNA – anti-double stranded DNA; PTT-LA – prolonged partial thromboplastin time-lupus anticoagulant.
Repeat testing of PTT-LA 12 weeks later revealed a similar result, confirming the diagnosis. The patient did not meet the Systemic Lupus International Collaborating Clinics classification criteria for SLE nor the new European League Against Rheumatism and American College of Rheumatology SLE classification criteria because the acute neurological symptoms were attributed to CVST [10]; however, he met the consensus criteria for APS [8]. Therefore, he was diagnosed with primary APS and hyperhomocysteinemia and started on folic acid and vitamin B-complex in addition to his previous treatment. After 1 month of treatment, there was no improvement in his ocular condition or vision, even though the anticoagulation target was achieved; therefore, he consented to undergo a right ONSF.
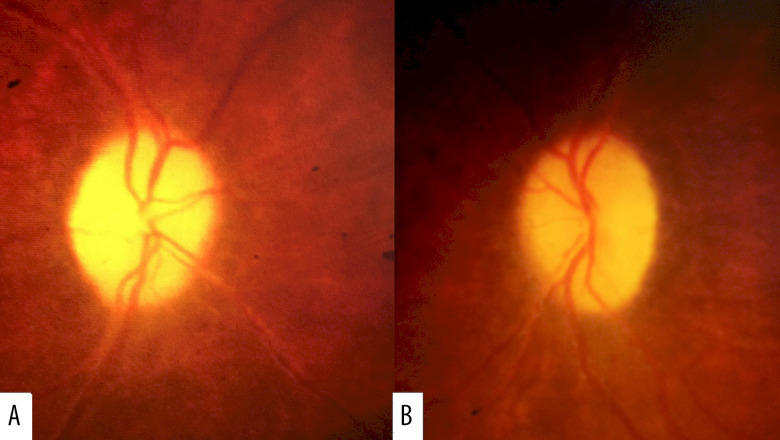
The surgery was subsequently performed through a medial transconjunctival approach, with no serious intraoperative or postoperative complications. One month later, CDVA improved to 20/200 in both eyes and posterior segment examination revealed resolution of the papilledema with persistent bilateral optic disc pallor (Figure 4A, 4B). Follow-up 6 months later revealed continued improvement in the patient’s condition, with 20/100 and 20/150 vision in his right and left eyes, respectively, and no new systemic manifestations. He continues to be monitored regularly for the development of SLE manifestations while being maintained on anticoagulant treatment with a target INR of 2 to 3.
Figure 4.
One month after right optic nerve sheath fenestration, posterior segment examination revealed resolution of papilledema in the A: right and B: left eyes with persistent optic disc pallor.
Discussion
The reason for CVST in our patient is not completely clear, but several etiologies are possible. Although he had SLE-specific antibodies as well as antiphospholipid antibodies, he did not meet the diagnostic criteria for SLE. It is important to note, however, that patients with primary APS can have antibodies that are specific for SLE, including anti-dsDNA and anti-nucleosome antibodies, without having SLE manifestations [11]. These patients, however, are at increased risk of developing SLE in the future [11]. Other patients with APS may develop some manifestations of SLE, known as lupus-like disease, without developing the full criteria for SLE diagnosis [11]. We believe that our patient falls into 1 of these categories and, in addition to requiring prolonged treatment for his APS, also requires monitoring for development of future SLE manifestations.
It is important to differentiate between primary APS and secondary APS associated with SLE because the treatment for the conditions is different and the latter may require prolonged treatment with steroids and immunosuppressive agents, which can be associated with increased morbidity, including multiple ocular and systemic adverse effects. As a result, we decided to monitor our patient for the development of SLE manifestations and the fulfillment of the diagnostic criteria for it before initiating such treatment, while maintaining anticoagulant therapy [8,10].
Reported ophthalmic manifestations associated with APS include retinal vessel thrombosis, amaurosis fugax, diplopia, hemianopsia, and papilledema [12]. Papilledema in APS mainly occurs due to CVST, which results in elevation in cerebrospinal fluid (CSF) pressure and can present as isolated intracranial hypertension, with papilledema being the main manifestation, leading to optic atrophy and blindness [6]. The main treatment for APS is anticoagulation rather than immunosuppression [13]. Patients with arterial disease or recurrent events or both may require aggressive treatment with a target INR of >3; however, patients with non-life-threatening thrombosis can receive less intense treatment [13].
Hyperhomocysteinemia is characterized by elevated plasma homocysteine due to interaction of genetic and environmental determinants, which results in increased risk of DVT, including a 4-fold increased risk of CVST that was found in a single study, and which could interact with other risk factors for DVT, such as use of oral contraceptives [3]. Folic acid and vitamin B-complex supplementation may help lower the plasma level of homocysteine; however, it may not lower the risk of thrombosis.
It is important to perform MRV in all patients with papilledema to exclude CVST, especially those not typically at high risk for IIH, such as non-obese men [14]. Indications for ONSF for the treatment of papilledema associated with IIH are currently unclear but include CDVA <0.8 in the better-seeing eye, progressive loss of vision or progressive visual field changes, and failure of medical treatment [15,16]. Previously, ONSF has been reported to be safe and effective for treatment of papilledema associated with CVST that is unresponsive to medical treatment; however, caution should be exercised during surgery because of the increased risk of bleeding due to the systemic anticoagulation that is used in these cases [17]. That reported case, however, was not associated with APS. ONSF can result in decreased CSF pressure around the optic nerve and resolution of papilledema by allowing continuous drainage of the CSF through fistula formation or formation of scar tissue, which prevents the elevated CSF pressure from being transmitted to the optic nerve head [18]. Unilateral ONSF has been reported to be effective for relief of bilateral papilledema associated with IIH; therefore, unilateral surgery may also suffice in cases associated with CVST, as it has in the present case [19].
Conclusions
In conclusion, ONSF may be safe and effective for treatment of papilledema associated with CVST that is refractory to medical treatment. Future studies are needed to better define the role of this modality and provide more information about its safety and effectiveness.
Footnotes
Ethics Approval
This report was approved by the Cairo University Research Ethics Committee and follows the tenets of the Declaration of Helsinki.
Conflict of Interest
None.
References:
- 1.Ehtisham A, Stern BJ. Cerebral venous thrombosis: Areview. Neurologist. 2006;12(1):32–38. doi: 10.1097/01.nrl.0000178755.90391.b6. [DOI] [PubMed] [Google Scholar]
- 2.Mokri B, Jack JR CR, Petty GW. Pseudotumor syndrome associated with cerebral venous sinus occlusion and antiphospholipid antibodies. Stroke. 1993;24(3):469–72. doi: 10.1161/01.str.24.3.469. [DOI] [PubMed] [Google Scholar]
- 3.Martinelli I, Battaglioli T, Pedotti P, et al. Hyperhomocysteinemia in cerebral vein thrombosis. Blood. 2003;102:1363–66. doi: 10.1182/blood-2003-02-0443. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Chen H, Zhang Y, et al. Clinical characteristics of cerebral venous sinus thrombosis in patients with systemic lupus erythematosus: A single-centre experience in China. J Immunol Res. 2015;2015:540738. doi: 10.1155/2015/540738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y, Tian X, Wang X. Diagnosis and treatment of cerebral venous thrombosis: A review. Front Aging Neurosci. 2018;10:2. doi: 10.3389/fnagi.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537–42. doi: 10.1212/wnl.53.7.1537. [DOI] [PubMed] [Google Scholar]
- 7.Li K, Ren M, Meng R, et al. Efficacy of stenting in patients with cerebral venous sinus thrombosis-related cerebral venous sinus stenosis. J Neurointerv Surg. 2019;11(3):307–12. doi: 10.1136/neurintsurg-2018-014328. [DOI] [PubMed] [Google Scholar]
- 8.Keeling D, Mackie I, Moore GW, et al. British Committee for Standards in Haematology Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 9.Fritsma GA, Dembitzer FR, Randhawa A, et al. Recommendations for appropriate activated partial thromboplastin time reagent selection and utilization. Am J Clin Pathol. 2012;137(6):904–8. doi: 10.1309/AJCP3J1ZKYBFQXJM. [DOI] [PubMed] [Google Scholar]
- 10.Dahlström Ö, Sjöwall C. The diagnostic accuracies of the 2012 SLICC criteria and the proposed EULAR/ACR criteria for systemic lupus erythematosus classification are comparable. Lupus. 2019;28(6):778–82. doi: 10.1177/0961203319846388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreoli L, Pregnolato F, Burlingame RW, et al. Antinucleosome antibodies in primary antiphospholipid syndrome: A hint at systemic autoimmunity? J Autoimmun. 2008;30(1-2):51–57. doi: 10.1016/j.jaut.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Franco AMM, Medina FMC, Balbi GGM, et al. Ophthalmologic manifestations in primary antiphospholipid syndrome patients: A cross-sectional analysis of a primary antiphospholipid syndrome cohort (APS-Rio) and systematic review of the literature. Lupus. 2020;29(12):1528–43. doi: 10.1177/0961203320949667. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498–509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- 14.Peralta GM, Cestari DM. An update of idiopathic intracranial hypertension. Curr Opin Ophthalmol. 2018;29(6):495–502. doi: 10.1097/ICU.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 15.Elnahry GA, Elemary AM, Badr Eldin N, et al. Peripapillary microperimetry for the diagnosis and follow-up of papilledema in cases treated for idiopathic intracranial hypertension. Neurol Res. 2021;43(1):61–70. doi: 10.1080/01616412.2020.1820811. [DOI] [PubMed] [Google Scholar]
- 16.Elnahry AG, Elnahry GA. Management of idiopathic intracranial hypertension during the COVID19 pandemic. Rev Recent Clin Trials. 2020. [Online ahead of print] [DOI] [PubMed]
- 17.Murdock J, Tzu JH, Schatz NJ, Lee WW. Optic nerve sheath fenestration for the treatment of papilledema secondary to cerebral venous thrombosis. J Neuroophthalmol. 2014;34(1):67–69. doi: 10.1097/WNO.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 18.Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral non-operated eyes in idiopathic intracranial hypertension. Ophthalmology. 2011;118(2):412–14. doi: 10.1016/j.ophtha.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Shuaib MM, Helmy GM, Badr Eldin N, et al. Optical coherence tomography of the optic nerve head before and after optic nerve sheath fenestration for idiopathic intracranial hypertension. Acta Neurol Belg. 2020;120(3):775–77. doi: 10.1007/s13760-020-01331-4. [DOI] [PubMed] [Google Scholar]