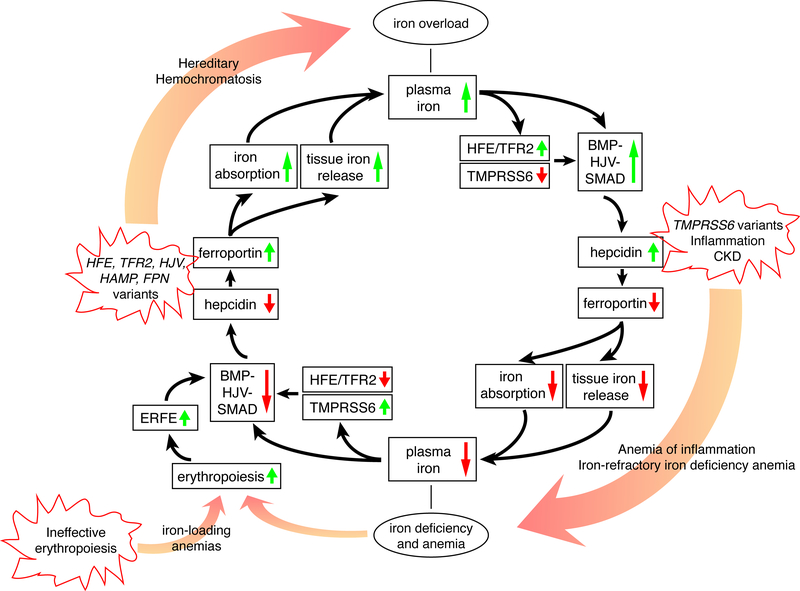
Figure 1. The iron regulatory cycle.
The inner circle with black arrows illustrates how iron homeostasis is regulated by the hepcidin-ferroportin axis. Starting from the top, high plasma and/or tissue iron leads to the activation of the HFE/TFR2 system and the BMP/HJV/SMAD signaling pathway that upregulate hepcidin expression. Hepcidin degrades ferroportin which limits iron absorption and tissue iron release, thereby lowering plasma iron concentration (bottom). Starting from the bottom, iron deficiency activates TMPRSS6 and suppresses the HFE/TFR2 and BMP/HJV/SMAD signaling pathway, thereby lowering hepcidin expression, increasing ferroportin activity, and increasing iron absorption from the diet and mobilization from body stores to increase plasma iron. Anemia (bottom) stimulates erythropoiesis. Proliferating red blood cell precursors secrete ERFE that inhibits BMP/SMAD signaling to lower hepcidin and increase iron availability. The outer circle of this figure displays how genetic variants or diseases lead to iron deficiency or overload. In hereditary hemochromatosis (HH), genetic variants in HFE, TFR2, HJV, HAMP or FPN (gain of function) downregulate hepcidin and/or activate ferroportin, which eventually lead to iron overload. TMPRSS6 inactivation, inflammation or CKD upregulate hepcidin, leading to iron refractory iron deficiency anemia (IRIDA) or anemia of inflammation (AI). Ineffective erythropoiesis in iron-loading anemias such as β-thalassemia leads to excessive ERFE production, hepcidin downregulation and iron overload.