Fig. 8.
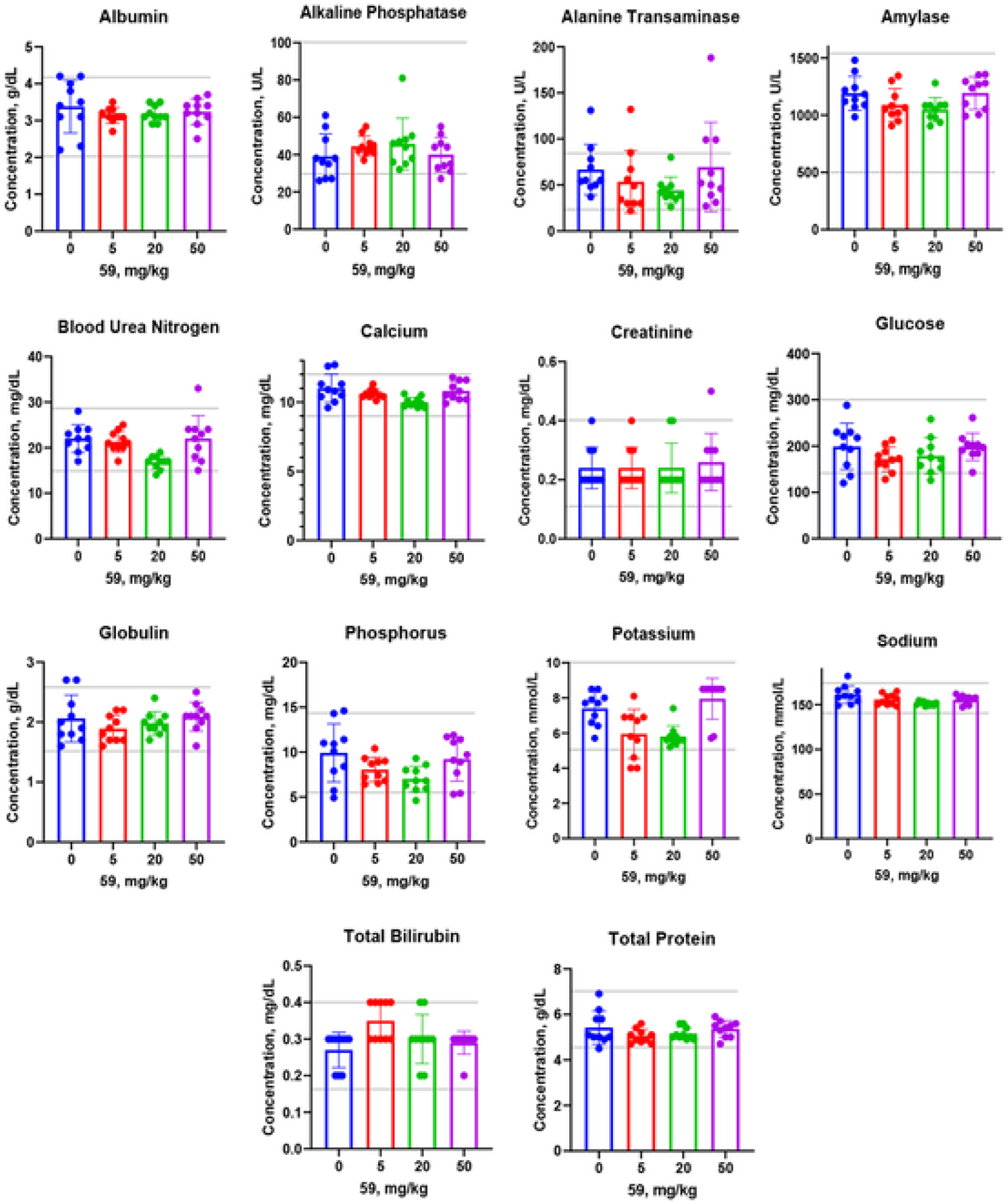
In vivo toxicity assessment of the compound 59. Three doses (5, 20 and 50 mg/kg) were administered IP in CD-1 mice (n = 10 per dose). Blood samples were collected 24 h later, and plasma samples were tested by 14 parameters for organ functions. Data are presented as Mean ± SE. Normal ranges are shown by gray horizontal lines. None of the doses show any statistically significant difference compared with vehicle control (0 mg/kg dose in saline).
