Abstract
The impact of the COVID-19 pandemic on dermatology practice cannot be overstated. At its peak, the pandemic resulted in the temporary closure of ambulatory sites as resources were reallocated towards pandemic response efforts. Many outpatient clinics have since reopened and are beginning to experience a semblance of pre-pandemic routine, albeit with restrictions in place. We provide an overview of how COVID-19 has affected dermatology practice globally beginning with the rise of teledermatology. A summary of expert recommendations that shape the “new normal” in various domains of dermatology practice, namely, dermatology consultation, procedural dermatology, and phototherapy, is also provided.
Keywords: COVID-19, Dermatology practice, Guidelines, New normal, Pandemic, Phototherapy, Procedural dermatology, Teledermatology
Key points
-
•
Dermatology practice adjustments during the COVID-19 pandemic have involved measures to facilitate physical distancing and curtail viral transmission.
-
•
Telemedicine utilization has increased tremendously and has continued to account for a significant proportion of overall visits even as clinics began to reopen.
-
•
Face-to-face consultations are unlikely to be replaced entirely by teledermatology, particularly for conditions that require closer inspection/palpation, microscopy, or biopsy.
-
•
During the early phases of the COVID-19 pandemic and associated lockdowns, dermatology procedures declined dramatically and were limited mostly to nonelective surgeries, whereas cosmetic procedures became exceedingly rare.
-
•
The decision to resume phototherapy should be made based on the weight of its perceived benefit versus the potential risks to both the patient and staff. Until widespread vaccination has been rolled out, patients may opt to forego phototherapy sessions and risk disease flares over fears of contracting COVID-19.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has substantially impacted medical practice worldwide. At its peak, lockdown measures were implemented in an effort to curb viral spread and reallocate resources and manpower toward the pandemic response. This situation entailed the closure of ambulatory sites that are deemed nonessential, which included dermatology outpatient clinics. As clinics began to reopen, dermatologists were faced with the challenge of navigating clinical practice while adhering to enhanced safety protocols (ie, physical distancing, mask wearing, frequent hand washing), and teledermatology, often referred to as the “new normal.” In this article, we describe how the COVID-19 pandemic has restructured the practice of dermatology and provide a summary of expert guidelines on the safe conduct of dermatology consultations, procedures, and phototherapy in the midst of this global health crisis.
The Rise of Teledermatology
During the height of the COVID-19 pandemic, many workers switched to working remotely to minimize in-person encounters and limit viral transmission. The medical field was no exception, as face-to-face patient encounters have been minimized to reduce the need for personal protective equipment (PPE) in short supply, whereas telemedicine was maximized. Telemedicine is defined as “the use of electronic information and communications technologies to provide and support health care when distance separates the participants.”1 This definition encompasses radio dispatching of emergency personnel, robotic surgery, and telephone and/or video consults.1 Being a highly visual field, dermatology is a field well suited to maximize telemedicine. The term “teledermatology” has been used to describe the use of telemedicine to evaluate skin lesions, review laboratory findings, and diagnose and treat patients remotely.2
First developed during the 1960s, the practice of teledermatology has increased exponentially in recent years.2 Teledermatology has proved to be vital during the peak of COVID-19 restrictions and, even as clinics have reopened, teledermatology continued to account for a significant proportion of overall dermatology visits.3 A recent analysis of trends in teledermatology use found that from May 2020 to June 2020, teledermatology consults for common dermatoses (ie, acne, rosacea, psoriasis, atopic dermatitis, and eczema) increased, whereas consults for skin malignancies decreased.3 This finding indicates that despite the availability of in-person consultation as an option, both patients and physicians felt comfortable addressing benign skin conditions via teledermatology.3 It is therefore reasonable to expect the long-term integration of telemedicine into dermatology practice, which necessitates the development of guidelines for optimal delivery of this service (Box 1 ).
Box 1. American Telemedicine Association clinical practice guidelines for teledermatology.
|
|
|
|
|
Data from McKoy K, Antoniotti NM, Armstrong A, et al. Practice Guidelines for Teledermatology. Telemed J E Health. 2016;22(12):981–90.
Teledermatology aims to improve access and accessibility to care, increase efficiency, and reduce cost2 , 4; however, it also has limitations. These limitations include technical difficulties (ie, poor Internet connection), privacy concerns, patient challenges with technology, access to technology, and lack of insurance coverage.5 , 6 In addition, there is potential for misdiagnosis due to incomplete history taking, poor photograph/video quality, and inability to perform physical examination (eg, lesion palpation) and diagnostic procedures.6 One review reports that more than half of teledermatology consultations require a subsequent in-person visit.2 Hence, clinicians must assess the appropriateness of teledermatology on a case-to-case basis.7
In-Person Consultation
Trends in average weekly patient visits during the initial phase of the pandemic (mid-February to mid-April) showed an 81% decline (from 149.7 to 28.2), with an uptick observed in mid-May (96.5 patients seen per week), commensurate with the gradual easing of lockdown restrictions in the United States8; this means that from February to May 2020, a potential 10.2 million patient visits were missed, which equates to an estimated decrease in revenue of $2.3 billion.8 In addition, a global Web-based survey of 733 dermatologists revealed that in-person consultation decreased by 54% following the onset of the pandemic, whereas teledermatology use increased 3-fold.9 More than two-thirds of survey respondents expect continued use of teledermatology in the future, further emphasizing its role in dermatology practice beyond the pandemic.9
Nonetheless, despite its increasing acceptability among both patients and practitioners alike, it is unlikely for teledermatology to entirely replace traditional face-to-face consultation. One study found that when presented with the same patient, there was a high degree of concordance (72%) between the diagnosis made by a dermatologist through teleconsultation and another dermatologist through face-to-face visit.10 However, it was also noted that 20% of the patients were deemed unfit for teleconsultation. These patients included those with conditions that cannot be sufficiently diagnosed without closer inspection and palpation, dermoscopy, fungal or viral microscopy, and biopsy.10 Hence, dermatology practice during the “new normal” involves determining whether a patient is suitable for teledermatology or in-person consultation.
Dermatology practices generally fall under the low-risk category for COVID-19 exposure.11 However, according to a study by Gerami and Liszewski,12 a dermatologist is likely to encounter 1 active COVID-19 case per week in the outpatient clinic, given an average of 165 new COVID-19 cases a day in a population of 100,000. Hence, during the pandemic, it is still prudent to have administrative and engineering measures in place to ensure the safety of both patients and staff. The American Academy of Dermatology recommended steps for running dermatology practice during the COVID-19 pandemic, first shared on their Web site in December 2020 (Box 2 ).11
Box 2. Steps for running dermatology practice during the coronavirus disease 2019 pandemic.
|
|
|
|
|
|
|
|
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Data from American Academy of Dermatology. Running Dermatology Practices During COVID-19. 2020. Available at: https://assets.ctfassets.net/1ny4yoiyrqia/1VQd8nAZqLCiLe7fGNlXrQ/230fd02e0b8d908b84905e57765ff57f/Running_Practices_During_Covid-19_12.03.20.pdf. Accessed February 12, 2021.
Most interim guidelines, when COVID-19 community spread was high, recommended seeing only urgent and essential cases, decreasing opening days and/or hours, reducing the number of staff per shift, and limiting the number of patients seen per day. Intervals in between appointments were lengthened, whereas the actual patient encounter was limited to as little time as possible (10–15 minutes). With appointment slots limited, triaging of patients for scheduling of in-person consultation became a necessity. Guidelines for prioritizing limited in-person appointments during the height of the pandemic suggested that precedence should be given to the following13:
-
•
Health care workers with skin diseases that interfere with their delivery of service.
-
•
Patients with severe skin diseases that are potentially life-threatening, functionally debilitating, or cause significant impairment to quality of life.
-
•
Diagnostic procedures for confirmatory purposes, especially when the differential diagnosis includes high-risk conditions (eg, melanoma, severe infection, mycosis fungoides, autoimmune blistering diseases).
-
•
Patients with skin disease resulting in significant functional and/or emotional impairment who have no access to or cannot effectively use telemedicine.
-
•
Patients with similar prognoses should be selected randomly as to who gets a particular appointment.
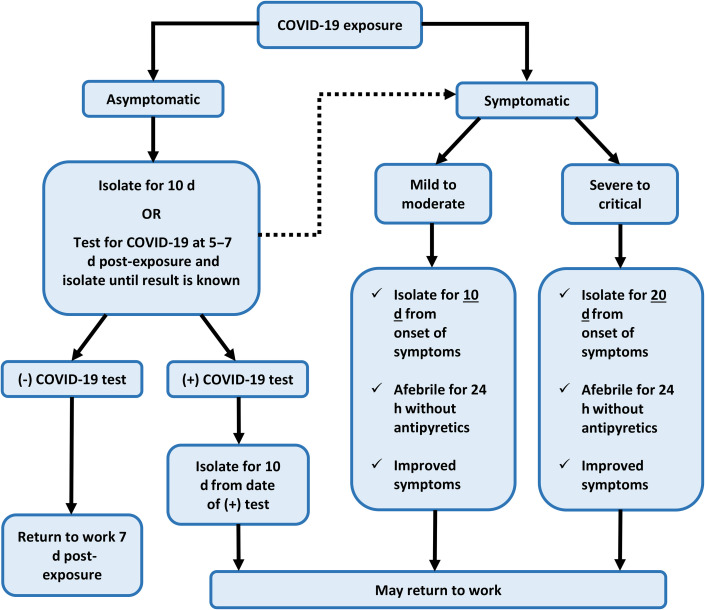
In addition, COVID-19 screening (temperature and symptom check) and wearing of masks became a routine, and in most cases even a prerequisite for a patient or staff member to be allowed entry into the clinic. It is recommended that staff members who are suspected to have COVID-19, either through positive screening or exposure to an infected individual, be sent home and follow the Centers for Disease Control and Prevention (CDC) guidelines for returning to work following a COVID-19 exposure (Fig. 1 ).14 Overall, these adjustments were made to facilitate physical distancing and curtail viral transmission.
Fig. 1.
Summary of CDC return to work criteria for health care staff who have been exposed to COVID-19. Exposure through close contact is defined by the CDC as being within 6 ft of an infected individual for at least 15 minutes without PPE. A previously asymptomatic staff member who starts to develop symptoms during 10-day isolation or while waiting for COVID-19 test results (dotted arrow) should follow the algorithm for symptomatic health care workers. Per CDC, fully vaccinated individuals (ie, ≥ 2 weeks and < 3 months from receiving requisite vaccine doses) or who have recovered from COVID-19 infection less than 3 months earlier do not have to quarantine after a meaningful COVID-19 exposure as long as they remain asymptomatic.
Data from Centers for Disease Control and Prevention (CDC). Return to work criteria for healthcare personnel with SARS-CoV-2 infection (interim guidance). 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fhcp-return-work.html. Accessed February 27, 2021.
Procedural Dermatology
Based on a global Web-based survey, only 25% of dermatologists performed procedures during the height of the pandemic.9 Of these, biopsies and Mohs micrographic surgeries (MMSs) were the most commonly performed, whereas cosmetic procedures became exceedingly rare,9 which comes as no surprise, because most interim guidelines in 2020 recommended deferring elective cosmetic and surgical procedures to reduce the risk of COVID-19 transmission and preserve PPE. The International League of Dermatologic Societies defines elective dermatologic procedures as those performed on skin lesions that pose no imminent danger to the patient if not surgically removed within 3 months15; these include acne surgery, chemical peels, laser hair removal, and injectables (botulinum toxin and cosmetic filler injections). Conversely, lesions such as melanoma, atypical melanocytic lesions, or abscess drainage may necessitate prompt management with surgery or other procedures, which should be done during the pandemic under strict infection prevention and control measures (see Box 2).
With regard to MMS, a United Kingdom-based nationwide survey revealed that almost half of surgeons performing MMS completely ceased services during the height of the pandemic, whereas 36% and 15% had reduced and normal operations, respectively.16 To minimize patient visits, those who continued to perform MMS showed an increased preference toward the use of absorbable sutures for wound closure, as well as telecommunications (telephone/video) for follow-up visits compared with before COVID-19.16 On the other hand, post-Mohs reconstructions performed by other specialties were significantly decreased (74%) together with face-to-face consultations (91% decrease).16
In early 2021, the American Society for Dermatologic Surgery together with the American Society for Laser Medicine and Surgery, Inc, released guidelines for the safe practice of cosmetic dermatology during COVID-19 (Table 1 ).17 The document detailed and graded ancillary evidence on various infection prevention and control measures (eg, mask/respirator use, eye protection, and handwashing), as well as the risk of viral transmission associated with certain dermatologic procedures.17
Table 1.
American Society of Dermatologic Surgery Association and American Society for Laser Medicine and Surgery guidelines for cosmetic dermatology practice during the coronavirus disease 2019 pandemic
| Recommendation | Level of Evidence | Strength of Recommendation |
|---|---|---|
| Use of masks by patients, physicians and staff | Moderate | Strong |
| Physician and staff masking for procedures near the nose and mouth | Moderate | Strong |
| Handwashing | Moderate | Strong |
| COVID-19 vaccination | Moderate | Strong |
| Eye protection | Moderate | Moderate |
| Use of air suction or HEPA filters | Moderate | Weak/Option |
| Use of upper-room UVGI | Moderate | Weak/Option |
| During prolonged skin procedures, properly fitted N95 respirators are a more effective form of protection than masks | Low | Weak/Option |
| Room size can influence the risk of COVID-19 infection (ie, larger rooms are associated with lower risk) | Low | Weak/Option |
| Longer patient contact time increases the risk of contracting COVID-19 | Low | Weak/Option |
| Procedures involving the head and neck carry greater risk of COVID-19 transmission compared with procedures below the clavicle | Low | Weak/Option |
| Forced air cooling increases the risk of COVID-19 transmission vs contact cooling during laser procedures | Very low | Weak/Option |
| Skin and hair procedures carry low risk of COVID-19 transmission | Very low | None |
| No documented risk of contracting COVID-19 from blood during procedures | Very low | None |
| No evidence that ablative laser procedures or liposuction increase the risk of COVID-19 infection | Very low | None |
Abbreviations: ASDSA, American Society of Dermatologic Surgery Association; ASLMS, American Society for Laser Medicine and Surgery; HEPA, high-efficiency particulate air; UVGI, ultraviolet-C germicidal irradiation.
Data from Narla S, Alam M, Ozog D, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) Guidance for Cosmetic Dermatology Practices During COVID-19. 2021. Available at: https://www.aslms.org/docs/default-source/for-professionals/resources/asdsa-and-aslms-final-cosmetic-reopening-guidance-june2020.pdf?sfvrsn=c879e53b_2. Accessed February 12, 2021.
Phototherapy
The COVID-19 pandemic significantly impacted the use of chronic dermatologic treatments, including phototherapy. Many phototherapy centers worldwide were closed during the height of the pandemic, whereas the few that remained open experienced a decline in patient census. In one of the biggest health systems in Israel, the number of patients coming in for phototherapy decreased by more than 50% since March 2020.18 This decrease was found to be primarily driven by patients declining treatment continuation because of fear of contracting the virus; the interruption in care posed the risk of a skin disease flare.18 Photoimmunosuppression may also be of particular concern amid the pandemic, because it is one of the mechanisms by which phototherapy controls skin disease. However, based on clinical experience with human immunodeficiency virus-positive patients, phototherapy is a safe and reasonable option during this time.19
The risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in phototherapy units is currently unknown.20 Although safety protocols observed in other hospital units are largely applicable, there are certain elements unique to phototherapy that require special attention. First, phototherapy involves having a patient come to the clinic multiple times a week, which potentially increases exposure to both the patient and staff. Second, localized treatments (ie, excimer laser or light) entail close contact between the patient and the staff for a prolonged period, and treatment of the face and periorificial areas where patients need to be unmasked puts the staff at even higher risk. Third, full-body treatments, although generally preferred during the pandemic, are typically administered in enclosed booths where patients stand in close proximity to phototherapy equipment surfaces made of plastic or steel.21 This proximity can potentially facilitate viral transmission because SARS-CoV-2 has been found to survive for up to 9 days on these surfaces,21 even though evidence of COVID-19 transmission through inanimate objects is limited. In addition, phototherapy booths normally have fans that are turned on during treatment to prevent overheating, which is potentially aerosolizing and could facilitate viral spread.22
Therefore, the decision to resume phototherapy should be made based on the weight of its perceived benefit versus the potential risks to both patient and staff. Most guidelines recommend prioritizing patients with severe skin disease, those who are more likely to respond to phototherapy, and in cases wherein other options besides phototherapy are limited or unavailable.20 Home phototherapy is also a reasonable option and may even be preferable during this time; however, it may not be feasible for all patients.
If in-office phototherapy is deemed necessary, efforts must be taken to conduct operations as safely as possible. Box 3 lists expert recommendations for operating phototherapy clinics during the COVID-19 pandemic.22 , 23
Box 3. Recommendations for phototherapy during the coronavirus disease 2019 pandemic.
|
|
Data from Lim HW, Feldman SR, Van Voorhees AS, Gelfand JM. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83(1):287–88 and Laconico-Tumalad LL, Sabido PWM, Sison-de Jesus C. Philippine Dermatological Society Photodermatology Subspecialty Core Group Post-Quarantine Guidelines for Phototherapy Centers. 2020. Available at: https://pds.org.ph/pds_new/wp-content/uploads/2020/06/PDS-Photodermatology-Post-ECQ-Guidelines-for-Phototherapy-FINAL.pdf. Accessed January 29, 2021.
Summary
| Before COVID | New Normal | |
|---|---|---|
| Dermatology consultation |
|
|
| Dermatology procedures |
|
|
| Phototherapy |
|
|
Future Perspectives
As of February 2021, a total of 72.8 million doses of COVID-19 vaccine have been administered in the United States, most which were first given to health care workers (HCWs).24 In Israel, which was the first country to vaccinate most of their population, fully vaccinated HCWs (2 doses of the Pfizer BioNT vaccine) comprised only 2% of those who contracted COVID-19; this compared favorably to partially vaccinated (1 dose received) and unvaccinated HCWs who comprised 46% and 52% of infections, respectively.25 Hence, with HCWs almost universally vaccinated, it is reasonable to expect that some easing of restrictions may take place. Per CDC guidance as of March 2021, individuals who are at least 2 weeks and less than 3 months from receiving the requisite doses of vaccine, or who have recovered from COVID-19 infection less than 3 months earlier, do not have to quarantine after a meaningful COVID-19 exposure as long as they remain asymptomatic.26 , 27 However, given its unpredictable nature, COVID-19 resurgence is a possibility and may warrant reinstatement of administrative and engineering measures detailed herein. Experts advise that even after receiving 2 doses of COVID-19 vaccine, individuals must still wear masks and practice physical distancing until more information becomes available.28
Clinics care points
-
•
COVID-19 is a rapidly evolving situation with expert recommendations changing at an almost daily basis, therefore, dermatologists must update themselves periodically and make necessary adjustments in accordance with local, state, and federal guidelines and mandates. The points summarized herein represent expert recommendation at the height of the pandemic.
-
•
The appropriateness of teledermatology must be assessed on a case to case basis. Many patients and dermatologists feel comfortable using teledermatology to address common dermatoses; however, lesions which require closer examination (i.e. suspected malignancy) may warrant a face-to-face visit.
-
•
Triaging of patients for scheduling in-person visits may be necessary. COVID-19 screening, wearing of masks, and physical distancing should still be practiced.
-
•
Elective procedures should be deferred in order to reduce the risk of COVID-19 transmission and preserve personal protective equipment, while necessary procedures should be done under strict infection control and prevention measures.
-
•
Many patients are reluctant to resume phototherapy for fear of contracting COVID-19. Home phototherapy, if feasible, is a reasonable option. Otherwise, in-office therapy should be resumed based on the perceived benefit versus potential risks, and should be conducted with safety protocols in place.
-
•
Despite health care workers almost universally vaccinated, COVID-19 resurgence is still a possibility. Experts recommend that fully-vaccinated individuals continue to wear masks and practice physical distancing until more information becomes available.
-
•
As the pandemic winds down, many of these recommendations/precautions can be safely relaxed.
Disclosures
Dr Torres has no relevant disclosures. Dr Ozog is an investigator for Biofrontera. Dr Hruza has no financial disclosures. He is Chair of the American Academy of Dermatology COVID-19 Ad-Hoc Task Force.
References
- 1.Field M.J. National Academies Press (US); Washington, DC: 1996. Telemedicine: a guide to assessing telecommunications in health care. [PubMed] [Google Scholar]
- 2.Romero G., Garrido J.A., Garcia-Arpa M. [Telemedicine and teledermatology (I): concepts and applications] Actas Dermosifiliogr. 2008;99(7):506–522. [PubMed] [Google Scholar]
- 3.Su M.Y., Smith G.P., Das S. Trends in teledermatology use during clinic reopening after COVID-19 closures. J Am Acad Dermatol. 2021;84(4):213–214. doi: 10.1016/j.jaad.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landow S.M., Mateus A., Korgavkar K., et al. Teledermatology: key factors associated with reducing face-to-face dermatology visits. J Am Acad Dermatol. 2014;71(3):570–576. doi: 10.1016/j.jaad.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R., Ibraheim M.K., Doan H.Q. Teledermatology in the wake of COVID-19: Advantages and challenges to continued care in a time of disarray. J Am Acad Dermatol. 2020;83(1):168–169. doi: 10.1016/j.jaad.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng J.N., Cembrano K.A.G., Wanitphakdeedecha R., et al. The aftermath of COVID-19 in dermatology practice: What's next? J Cosmet Dermatol. 2020;19(8):1826–1827. doi: 10.1111/jocd.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKoy K., Antoniotti N.M., Armstrong A., et al. Practice guidelines for teledermatology. Telemed J E Health. 2016;22(12):981–990. doi: 10.1089/tmj.2016.0137. [DOI] [PubMed] [Google Scholar]
- 8.Litchman G.H., Marson J.W., Rigel D.S. The continuing impact of COVID-19 on dermatology practice: Office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84(2):576–579. doi: 10.1016/j.jaad.2020.08.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhargava S., McKeever C., Kroumpouzos G. Impact of covid-19 pandemic on dermatology practices: results of a web-based, global survey. Int J Womens Dermatol. 2021;7(2):217–223. doi: 10.1016/j.ijwd.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordal E.J., Moseng D., Kvammen B., et al. A comparative study of teleconsultations versus face-to-face consultations. J Telemed Telecare. 2001;7(5):257–265. doi: 10.1258/1357633011936507. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Dermatology Running Dermatology Practices During COVID-19. 2020. https://assets.ctfassets.net/1ny4yoiyrqia/1VQd8nAZqLCiLe7fGNlXrQ/230fd02e0b8d908b84905e57765ff57f/Running_Practices_During_Covid-19_12.03.20.pdf Available at: Accessed February 12, 2021.
- 12.Gerami P., Liszewski W. Risk assessment of outpatient dermatology practice in the setting of the COVID-19 pandemic. J Am Acad Dermatol. 2020;83(5):1538–1539. doi: 10.1016/j.jaad.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoff B.K., Blalock T.W., Swerlick R.A., et al. Guiding principles for prioritization of limited in-person dermatology appointments during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83(4):1228–1230. doi: 10.1016/j.jaad.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Return to Work Criteria for Healthcare Personnel with SARS-CoV-2 Infection (Interim Guidance) 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fhcp-return-work.html Available at: Accessed February 27, 2021.
- 15.International League of Dermatological Societies Guidance on the practice of dermatosurgery and cosmetic procedures during the COVID-19 (SARS-CoV-2, Coronavirus) pandemic (updated June 2020) 2020. https://ilds.org/wp-content/uploads/2020/06/ILDS-Guidance-on-the-practice-of-dermatosurgery-and-cosmetic-procedures-COVID-19-Update-June-2020.pdf Available at: Accessed February 12, 2021.
- 16.Nicholson P., Ali F.R., Mallipeddi R. Impact of COVID-19 on Mohs micrographic surgery: UK-wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45(7):901–902. doi: 10.1111/ced.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narla S., Alam M., Ozog D., et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) Guidance for Cosmetic Dermatology Practices During COVID-19. 2021. https://www.aslms.org/docs/default-source/for-professionals/resources/asdsa-and-aslms-final-cosmetic-reopening-guidance-june2020.pdf?sfvrsn=c879e53b_2 Available at: Accessed February 12, 2021.
- 18.Fisher S., Ziv M. COVID-19 effect on phototherapy treatment utilization in dermatology. J Dermatol Treat. 2020:1–3. doi: 10.1080/09546634.2020.1781043. [DOI] [PubMed] [Google Scholar]
- 19.Torres A.E., Lyons A.B., Hamzavi I.H., et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84(2):479–485. doi: 10.1016/j.jaad.2020.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguilera P., Gilaberte Y., Perez-Ferriols A., et al. Management of phototherapy units during the COVID-19 pandemic: Recommendations of the AEDV's Spanish Photobiology Group. Actas Dermosifiliogr. 2021;112(1):73–75. doi: 10.1016/j.ad.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tursen U., Tursen B., Lotti T. Ultraviolet and COVID-19 pandemic. J Cosmet Dermatol. 2020;19(9):2162–2164. doi: 10.1111/jocd.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim H.W., Feldman S.R., Van Voorhees A.S., et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83(1):287–288. doi: 10.1016/j.jaad.2020.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laconico-Tumalad L.L., Sabido P.W.M., Sison-de Jesus C. Philippine Dermatological Society Photodermatology Subspecialty Core Group Post-Quarantine Guidelines for Phototherapy Centers. 2020. https://pds.org.ph/pds_new/wp-content/uploads/2020/06/PDS-Photodermatology-Post-ECQ-Guidelines-for-Phototherapy-FINAL.pdf Available at: Accessed January 29, 2021.
- 24.Centers for Disease Control and Prevention (CDC) COVID-19 Vaccinations in the United States. 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations Available at: Accessed February 27, 2021.
- 25.Amit S., Regev-Yochay G., Afek A., et al. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) When to Quarantine. 2021. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html Available at: Accessed February 28, 2021.
- 27.Centers for Disease Control and Prevention (CDC) Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html Available at: Accessed February 28, 2021.
- 28.Centers for Disease Control and Prevention (CDC) Frequently Asked Questions about COVID-19 Vaccination. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html Available at: Accessed February 27, 2021.