Abstract
Biochar adsorbent can be produced in low-resource settings using local materials and simple pyrolysis technology, and it has shown promise for uptake of micropollutants (MPs) such as pesticides, pharmaceuticals, industrial compounds, and chemicals released from consumer goods present in water at ng/L to μg/L levels. Accordingly, the use of biochar in water treatment applications where granular activated carbon (GAC) is economically or logistically infeasible is gaining interest. Monitoring treatment systems for individual MPs require laboratory analytical techniques that are typically cost-prohibitive and impractical for low-resource settings. Therefore, identification of surrogate parameters(s) for adsorbent bed life that can be measured inexpensively and in the field is a high priority. Background dissolved organic matter (DOM) is ubiquitous in natural and anthropogenic waters at concentrations typically 1,000 to 100,000 that of MPs. Some constituents of DOM foul the adsorbent and reduce bed life for removal of target contaminants. Aromatic DOM foulants absorb ultraviolet light at a wavelength of 254 nm (UVA254). Because DOM fouling directly affects MP adsorption capacity and DOM is a bulk water parameter that can be quantified using relatively inexpensive and portable instruments, it could be exploited as a surrogate for monitoring biochar adsorber bed life under field conditions. The objective of this study was to quantify removal of MPs from waters containing different types and concentrations of background DOM (surface water, wastewater, dump leachate) and thus exhibiting different UVA254 breakthrough profiles in bench-scale column experiments. Breakthrough profiles of weakly to moderately adsorbing MPs, including herbicides, pharmaceuticals and personal care products, and perfluoroalkyl acids, were collected using biochars generated under different pyrolysis conditions and a commercial GAC as a performance benchmark. Optimal conditions for biochar water treatment include using biochar produced from wood at ≥850°C under slightly aerobic conditions, empty bed contact times of ≥30 min, and upstream treatment processes to reduce DOM. Relative UVA254 breakthrough (C/C0) up to 0.6–0.9 corresponded to ≥90% MP removal for most MP-water combinations studied.
Keywords: biochar water treatment; contaminants of emerging concern; engineering for developing communities, hygiene for development; low-cost sensors; sanitation; water
Introduction
Organic micropollutants (MPs) such as pesticides, pharmaceuticals, industrial compounds, and consumer waste breakdown products occurring at ng/L to μg/L levels impact water sources around the globe. Biochar is an adsorbent for MPs that can be generated from local surplus biomass using simple pyrolysis technology, providing a potentially cost-effective and environmentally sustainable alternative to commercial granular activated carbon (GAC) (Kearns et al., 2015b, 2019, 2020; Shimabuku et al., 2016; Thompson et al., 2016; Inyang and Dickenson, 2017). In some cases, biochar can be obtained for around one-sixth the cost of GAC (Thompson et al., 2016). Biochar is particularly applicable in decentralized water treatment in low-resource settings such as communities in the developing world (Kearns et al., 2014, 2015b). In addition to biochar production conditions, the design and operational specifications of fixed-bed biochar adsorbers strongly influence treatment efficacy. Accordingly, a major objective of this study is to provide practical quantitative guidance for optimizing biochar contactors with respect to granular biochar particle size, contact time, mitigating undesired effects of the background water matrix, and the potential for MP desorption.
An important challenge for biochar water treatment, particularly in low-resource settings, is monitoring to determine when biochar adsorption capacity for removal of contaminants of concern to desired levels has been exceeded. Two interacting factors complicate this challenge. One factor is that, given the wide variety of MPs entering the environment and the costly laboratory methods required for their quantitation, measuring individual compounds is infeasible. The second factor pertains to the background chemical diversity of water sources. Dissolved organic matter (DOM) is ubiquitous in natural and anthropogenic waters. Constituents of DOM compete with target MPs for adsorption sites and constrict and block adsorbent pores, a process termed fouling. DOM fouling impacts both MP adsorption capacity and kinetics in complex and time-dependent ways, making generalization between specific water-adsorbent-MP systems difficult (Knappe et al., 1999; Li et al., 2003a, 2003b; Corwin and Summers, 2012; Shimabuku et al., 2014, 2016; Anumol et al., 2015; Kennedy and Summers, 2015; Kennedy et al., 2015, 2017).
However, because DOM fouling directly affects MP adsorption capacity, and DOM is a bulk water parameter that can be quantified using relatively inexpensive and portable instruments, it could be exploited as a surrogate for monitoring biochar adsorber bed life under field conditions. Accomplishing this requires establishing the relationship(s) between adsorption of DOM foulants and sentinel MPs in model biochar fixed-bed adsorbers. Weakly adsorbing MPs are preferred choices as sentinels since they are expected to break through biochar columns before moderately and strongly adsorbing compounds and would therefore dictate the frequency of biochar regeneration or replacement. Thus, a second major objective of this study is to establish quantitative relationships between the removal of DOM foulants and sentinel MPs in fixed-bed contactors.
DOM is frequently quantified and characterized by the measurement of dissolved organic carbon (DOC), absorbance of ultraviolet light at 254 nm (UVA254), and a variety of DOM fluorescence indices such as total fluorescence and intensity of different excitation-emission peaks (Korak et al., 2014). Studies with activated carbon (AC) have shown that DOC is a relatively poor indicator of MP breakthrough in carbonaceous adsorbents (Anumol et al., 2015; Sgroi et al., 2018). In contrast, recent work has suggested that a select group of fluorescence parameters could provide a more sensitive spectroscopic surrogate than UVA254 for predicting MP breakthrough in GAC adsorbers (Anumol et al., 2015; Ziska et al., 2016; Shimabuku et al., 2017; Sgroi et al., 2018). However, relative to fluorescence-based methods, UVA254 instrumentation is currently less expensive and more field-ready, in particular for low-resource settings, and thus is the subject of the present study.
UVA254 is an indicator of DOM aromaticity (Weishaar et al., 2003). In contrast to bulk DOC, moderate to strong correlations between uptake of MPs and UV-absorbing DOM by ACs have been demonstrated (Zietzschmann et al., 2014b, 2016a, 2016b; Anumol et al., 2015). This is because UV-absorbing DOM is more aromatic than non-UV-absorbing DOM and so has greater affinity for the graphitic surfaces of carbonaceous adsorbents. UV-absorbing DOM therefore includes most of the subfractions of bulk DOM that are responsible for fouling (Weishaar et al., 2003; Zietzschmann et al., 2016b; Shimabuku et al., 2017; Sgroi et al., 2018).
A few studies have been conducted correlating removal of UVA254 and MPs in fixed-bed GAC systems with promising results (Velten et al., 2011; Zietzschmann et al., 2014a, 2016b; Anumol et al., 2015; Kennedy and Summers, 2015; Shimabuku et al., 2017; Sgroi et al., 2018). One study found a strong correlation between UVA254 and sulfamethoxazole (SMX) breakthrough from laboratory bench-scale biochar columns treating surface water (SW) (Greiner et al., 2018). GAC studies using water with different DOM character, for example, SW and wastewater (WW) effluent, have shown that consistent relationships can be observed between UVA254 and MP uptake by AC within, but not between, different water types (Anumol et al., 2015; Zietzschmann et al., 2016a; Sgroi et al., 2018). It is well established that DOM source and composition have a major influence on MP adsorption due to the diverse constituencies of foulants present in different waters. Identifying an approach to surrogate monitoring that works across a wide range of background water chemistries would be an important and valuable contribution to the field. However, so far, only one study has been able to superimpose GAC breakthrough curves for a few MPs collected in SW and WW effluent (Zietzschmann et al., 2016b). This was accomplished by normalizing column throughput to the UVA254 of a low-molecular-weight DOM isolate obtained by size-exclusion chromatography with online organic carbon detection (Zietzschmann et al., 2016b). It remains to be established whether and how UVA254-based monitoring could provide a surrogate for MP removal by biochar treatment of diverse source waters using inexpensive field methods. This study advances that aim.
This article provides a quantitative comparison between breakthrough of UVA254-DOM and weakly to moderately adsorbing MPs in biochar columns treating waters of different origin: SW, WW effluent, and dump leachate (LE). The waters and MP were chosen to encompass a diversity of background water chemistries and characteristic pollution scenarios—for example, SW impacted by herbicide runoff, WW containing pharmaceuticals and personal care products, and LE containing persistent organic pollutants released by breakdown of consumer wastes.
SW UVA254-MP breakthrough relationships were quantified for several biochars generated by conventional anaerobic pyrolysis (CAP) or copyrolysis thermal air (CPTA) activation and compared with a commercial GAC as an adsorption benchmark. Previous work has shown CPTA biochars to possess much greater MP adsorption capacity than CAP biochars in batch tests (Kearns et al., 2015b, 2019; Shimabuku et al., 2016). The effects of adsorbent particle size, contact time, DOM preloading, and DOM influent concentration on UVA254 and MP breakthrough were quantified in SW experiments for a representative wood-based high temperature (900°C) CPTA biochar (identified as D-FD-HWP-900; naming convention explained in “Materials and Methods” section) and compared with similar experiments using GAC. UVA254-MP breakthrough relationships were also quantified for D-FD-HWP-900 biochar and GAC in WW and LE.
UVA254-MP breakthrough relationships were determined in the laboratory using rapid small-scale column tests (RSSCTs). The RSSCT uses the concept of similitude to scale the adsorption process using dimensionless parameters developed from the dispersed-flow pore and surface diffusion model. There are two common RSSCT design approximations—proportional diffusivity (PD) and constant diffusivity (CD). The CD approach assumes that MP intraparticle diffusion kinetics does not depend on adsorbent particle size, whereas the PD approach assumes a linearly proportional dependence of MP intraparticle diffusion kinetics on adsorbent particle size. At this time, there is no clearly superior approach for all situations (Summers et al., 2014). RSSCT experiments are operated according to the RSSCT design equation [Eq. (1)]:
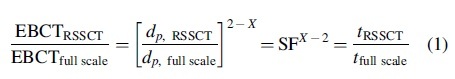
where EBCT stands for empty bed contact time, dp is particle diameter, SF is scaling factor, and t is operation time. For CD, the diffusivity factor X is equal to 0, whereas for PD it is equal to 1.
In this study, the CD-RSSCT was chosen for two reasons. One is that the CD-RSSCT presents even greater savings of time and experimental resources compared with the PD-RSSCT. This was important because especially in the case of WW and LE experiments very limited amounts of test waters were available. In addition, when high levels of MP removal (i.e., C/C0 ≅ 10%) are desired, which was the primary concern of this study, the CD approach is generally superior to the PD approach for predicting the onset of breakthrough (Summers et al., 2014).
Two methods were used to establish preliminary full-scale validation of RSSCT UVA254-MP breakthrough relationships determined in the laboratory. One, a modeling approach was used to predict a breakthrough curve for the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) for pairing with UVA254 breakthrough data collected from a full-scale biochar system treating SW on a farm in Thailand to compare with the observed SW RSSCT UVA254–2,4-D breakthrough relationship using the same biochar. Second, full-scale SMX and UVA254 breakthrough data were obtained from a GAC pilot column treating WW for comparison with the observed RSSCT breakthrough relationship using the same GAC and WW.
Materials and Methods
Adsorbents
Biochar generation conditions are reported in detail in previous studies (Kearns et al., 2015a, 2019, 2020). In brief, CPTA biochars were generated in natural draft (ND) and forced draft (fan assisted, FD) modes using one-gallon cookstove (C-) and 55-gallon drum oven (D-) gasification pyrolysis units over temperatures ranging from 625°C to 900°C. The feedstocks used were pine pellets (PINE), pecan shells (PEC), cherry pits (CHER), chopped eucalyptus (EUC), and hardwood pellets (HWP). The naming convention for biochars is as follows: “C” or “D” signifying cookstove or drum oven, followed by ND or FD for mode of operation, followed by the abbreviation denoting the feedstock and the peak pyrolysis temperature. For example, D-FD-HWP-900 signifies drum oven, forced draft, and hardwood pellets, 900°C. CAP biochars were generated from eucalyptus wood (W) cut into slats (15 × 10 × 1 cm) and placed in a metal retort (R), covered with sand to exclude oxygen, and heated using a programmable laboratory muffle furnace to 550°C, 700°C, or 850°C over a period of 8 h (denoted R-550, W-R-700, and W-R-850). Wood slats were also heated to 350°C for a period of 4 days (W-R-350-4d), and 600°C for a period of 3 days (W-R-600-3d). Characterization data for these biochars (elemental and ash content, surface area and porosimetry) were reported previously (Kearns et al., 2015a, 2019, 2020). The commercially available GAC used in this study was generated by steam activation of bituminous coal (Norit GAC 830, average particle diameter 1.285 mm), referred to in this article simply as “GAC.”
For column testing, biochar and GAC were ground by hand in a mortar and pestle to obtain the fraction retained between #100 and #200 United States standard sieves (average particle diameter, dp, 0.108 mm) for the majority of experiments. Particle diameters reported are log-mean values of upper and lower sieve sizes. To quantify the effect of particle size, D-FD-HWP-900 biochar and GAC were ground and sieved to obtain fractions between #80 and #100 and #200 and #325 United States standard sieves—average dp values of 0.165 and 0.059 mm, respectively. Laboratory grade organic-free (“distilled, deionized”) water was used to wet-sieve biochar and GAC to obtain desired size fractions for all column experiments.
Waters and adsorbates
SW experiments
RSSCT experimental data collected in three different waters are reported in this study. The model SW contained background DOM isolated from a SW source near Big Elk Meadows, Colorado, United States. This watershed is not impacted by agricultural runoff or WW discharge. It was held at pH 7 using 20 mM phosphate buffer (1.6 g/L KH2PO4 and 1.1 g/L Na2HPO4). For most SW experiments a DOM concentration of 4 mg/L DOC was used as this is representative of many SWs. The UVA254 of this water was 0.128 cm−1. To investigate the impact of DOM concentration, RSSCTs were also conducted with DOM concentrations of 2 mg/L, 1 mg/L, and nominally 0 mg/L (denoted “0 mg/L”) using laboratory reagent (DI) water. To determine MP uptake at environmentally relevant levels, 100 μg/L of 3H-labeled 2,4-D and 300 ng/L of 14C-labeled SMX or 1.5 μg/L of 14C-labeled simazine (SZN; American Radiolabeled Chemicals, Inc.) were introduced to the initial DOM solution matrix and quantified by liquid scintillation counting (method detection limit 1 μg/L for 3H-2,4-D, 10 ng/L for 14C-SMX, and 62 ng/L for 14C-SZN). The United States Environmental Protection Agency's maximum contaminant levels for 2,4-D and SZN in drinking water are 70 and 4 μg/L, respectively, whereas the World Health Organization Guideline Values are 30 and 2 μg/L, respectively. SMX is currently unregulated. RSSCTs in SW were conducted with GAC and all biochars listed above. Sodium azide (100 mg/L) was added to SW to inhibit MP biodegradation.
WW experiments
Tertiary-filtered WW effluent from a full-scale WW treatment facility in Las Vegas with pH 7.2 and DOM at a concentration of 4.9 mg/L DOC (UVA254 0.234 cm−1) was also used in RSSCT experiments. Full-scale treatment of WW involved conventional activated sludge treatment (secondary treatment) comprising a modified Johannesburg process for biological nitrogen and phosphorus removal followed by tertiary treatment by dual media (anthracite and sand) filtration. The WW contained 11 native MPs, which consisted of 10 pharmaceuticals and personal care products—atenolol, carbamazepine, N,N-diethyl-meta-toluamide, fluoxetine, meprobamate, naproxen, primidone, sucralose, SMX, and trimethoprim—and one flame retardant, tris(2-chloroethyl) phosphate. All native compounds ranged in concentration from a few tens to a few hundreds of ng/L except for SMX (∼1 μg/L) and sucralose (∼38 μg/L). Sodium azide (100 mg/L) was added to WW to inhibit MP biodegradation. 2,4-D and SZN were not natively present above detection limits in WW, and therefore were spiked as 3H-2,4-D and 14C-SZN at 100 μg/L and 1.5 μg/L to compare herbicide removal from WW and SW. Native MPs were analyzed by the Southern Nevada Water Authority. Samples were concentrated via automated solid phase extraction using a Dionex Auto Trace 280 workstation and analyzed via isotope-dilution liquid chromatography with tandem mass spectrometry (LC/MS-MS) using an API 4000 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). Radiolabeled MPs were analyzed by liquid scintillation counting as described above. RSSCTs in WW were conducted with GAC and D-FD-HWP-900 biochar.
Dump LE experiments
Approximately 100 mL of concentrated LE was obtained from an open, unlined municipal waste dump site in Sri Lanka. Concentrated LE was diluted using laboratory reagent water to obtain a solution with a DOM concentration of 135 mg/L DOC (UVA254 0.411 cm−1) for use in RSSCT experiments. This LE solution (abbreviated LE) was spiked with a mixture of seven perfluorocarboxylate compounds ranging in chain length from 4 to 10 carbons—perfluorobutanoate (PFBA), perfluoropentanoate (PFPeA), perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), and perfluorodecanoate (PFDA)—and two perflurosulfonate compounds, perfluorobutanesulfonate (PFBS) and perfluorooctanesulfonate (PFOS). PFAS reagents (Wellington Laboratories) were added to attain a concentration of 350 ng/L of each congener. Influent and effluent samples from RSSCTs were analyzed by LC/MS-MS. RSSCTs in LE were conducted with GAC and D-FD-HWP-900 biochar.
RSSCT design A
Two CD-RSSCT designs were used in this study. Design A, used in SW and WW experiments, was modeled on a full-scale biochar adsorber 58 cm in diameter, 30 cm in depth, with an average particle size of 4.5 mm and an EBCT of 2.5 h, and contained within a surplus 200 L (55 gal) high density polyethylene drum. Household and small community drinking water treatment systems incorporating these biochar contactors have been widely deployed in the developing world, in particular in SE Asia (Kearns et al., 2016). Because of the difficulty associated with processing large quantities of biochar to the smaller and more uniform granule sizes typical of commercial GACs in low-resource settings, a rather large average particle size (4.5 mm) is imputed here, and is compensated by a long contact time (EBCT 2.5 h). However, because mass transfer dimensionless groups scale with particle size, these conditions are equivalent to a GAC system using 12 × 40 United States standard mesh particles (average dp 0.92 mm) operating with a 30 min EBCT. In other words, if the RSSCT design equation [Eq. (1)] is solved for a contactor with an EBCT of 2.5 h using 4.5 mm diameter particles, the resulting dimensionless parameters that describe mass transfer will be the same as for a contactor operating with an EBCT of 30 min using 0.92 mm diameter particles. Both contactors are operating in “similitude” from a mass transfer perspective. A 30 min EBCT is long but within the acceptable range for drinking water and tertiary WW treatment systems. CD-RSSCT-A columns were constructed from refillable stainless steel high-performance liquid chromatography (HPLC) guard column hardware (Moeller Medical, Germany) with an internal diameter of 0.4 cm. To quantify MP desorption in SW, WW, and DI water experiments, when ∼90% 2,4-D breakthrough was attained, influent solutions were switched to the same matrix omitting MPs, and MP concentrations measured in column effluent over ∼20,000 bed volumes (BV).
RSSCT design B
CD-RSSCT design B was used for quantifying PFAS uptake from LE. RSSCT experiments were modeled on a full-scale adsorber with a bed diameter and bed depth of 1 m, using 8 × 30 United States standard mesh particles (average dp 1.29 mm), and with an EBCT of 90 min. Long (30–90 min.) EBCTs have been shown to be optimal for GAC systems treating landfill LE in a number of cases (Halim et al., 2010; Singh et al., 2012; Luo et al., 2020). Design B columns were constructed from polycarbonate tubing with an inner diameter of 0.47625 cm (3/16′′) and stainless-steel Swagelok fittings. Full-scale and CD-RSSCT column operational parameters are listed in Table 1 and Supplementary Table S1.
Table 1.
Full-Scale and Constant Diffusivity-Rapid Small-Scale Column Test Operational Parameters
| Parameter | Full-scale adsorber A | CD-RSSCT Design A | Full-scale adsorber B | CD-RSSCT Design B |
|---|---|---|---|---|
| EBCT | 152 min | 5.3 s | 90 min | 38.3 s |
| United States standard mesh size | N/A | 100 × 200 | 8 × 30 | 100 × 200 |
| Particle diameter | 4.5 mm | 0.108 mm | 1.29 mm | 0.108 mm |
| Column diameter | 58 cm | 4.0 mm | 1 m | 0.47625 cm |
| Bed length | 30 cm | 10 mm | 1 m | 8.42 cm |
| Flow rate | 0.52 L/min (750 L/day) | 1.43 mL/min | 8.73 L/min (12,570 L/day) | 2.35 mL/min |
| Adsorbent mass | 18 kg (biochar) | 61 mg (GAC), 28.5 mg (biochar) | 380 kg (GAC) | 730 mg (GAC), 340 mg (biochar) |
| Reynolds number | 0.43 | 0.43 | 0.69 | 0.69 |
| Biot number | 39 | 39 | 46 | 46 |
Biot and Reynolds numbers are shown to indicate similitude of mass transfer parameters between large- and small-scale columns. Also, Biot numbers indicate that mass transfer is dominated by intraparticle diffusion and that film diffusion makes only a small or negligible contribution to overall mass transfer rate (i.e., Biot numbers in the range of 5–100).
CD, constant diffusivity; EBCT, empty bed contact time; GAC, granular activated carbon; N/A, not applicable; RSSCT, rapid small-scale column test.
Results and Discussion
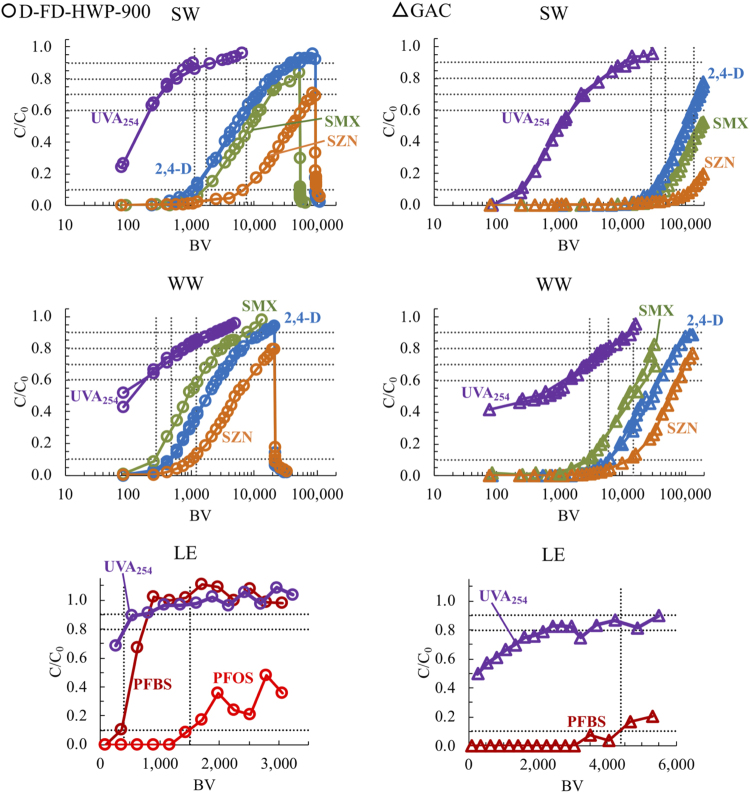
Figure 1 shows representative MP breakthrough curve data in SW, WW, and LE for D-FD-HWP-900 biochar and GAC. 2,4-D, SMX, and SZN breakthrough curves in SW for other biochars are provided in Supplementary Fig. S1b–l. MPs broke through in the order 2,4-D → SMX → SZN in all SW experiments, whereas SMX broke through before 2,4-D and the other WW MPs in WW experiments. Breakthrough curves for the other WW MPs are provided in Supplementary Fig. S2. Figure 1 shows PFBS and PFOS breakthrough in LE for D-FD-HWP-900 biochar, and PFBS breakthrough for GAC. The experiment was discontinued before the onset of PFOS breakthrough in the GAC column because of the limited volume of test water. Breakthrough curves of the other PFAS compounds from D-FD-HWP-900 and GAC columns are provided in Supplementary Fig. S3.
FIG. 1.
Representative UVA254 and micropollutant breakthrough curves for D-FD-HWP-900 biochar (circles) and GAC (triangles) in SW, WW, and LE. SW, surface water; WW, wastewater; LE, leachate; GAC, granular-activated carbon; UVA254, ultraviolet light at a wavelength of 254 nm.
In SW experiments with DOM at a concentration of 4 mg/L DOC, immediate MP breakthrough was observed for all CAP (W-R-350-4d, W-R-550, W-R-600-3d, W-R-700, and W-R-850; Supplementary Fig. S1h–l) and moderate temperature CPTA (C-ND-PINE-625 and C-ND-CHER-650; Supplementary Fig. S1f, g) biochars tested. This indicates that the mass transfer zone was not completely captured within the RSSCT for these adsorbents. MP mass transfer zones were captured in all high-temperature CPTA biochar (D-FD-HWP-900, C-FD-PINE-875, C-FD-PEC-900, C-FD-CHER-875, and D-ND-EUC-850) and GAC SW experiments (Fig. 1 and Supplementary Fig. S1b–e, m, n, p–r, and t–z). High-temperature (≥850°C) CPTA conditions appear to produce the most effective biochars for MP removal in fixed-bed contactors, which is consistent with results from batch studies (Kearns et al., 2015a, 2019). For SMX removal from SW by wood-based biochars, results of this study are similar to observations made by Greiner et al. (2018). SMX BV10% values in this study ranged from 1,540 to 5,850 BV, and ranged from ∼1,000 to 10,000 BV for fresh, regenerated, and enhanced biochars in Greiner et al.'s (2018) study.
In WW experiments, SMX was the first MP to break through in D-FD-HWP-900 and GAC columns (Fig. 1 and Supplementary Fig. S2). Mass transfer zones thus appeared to be captured for all WW MPs with these two adsorbents. Due to the volume of sample required for sufficient recovery and analysis of WW MPs by LC/MS-MS, the first effluent sample occurred at around 1,000 BV compared with around 100 BV for radiochemical analyses of SMX, 2,4-D, and SZN. Some WW MPs were quantified above MS detection levels in this sample (Supplementary Fig. S2). In these cases, it can only be inferred that the mass transfer zone was fully captured within the column because those MPs breakthrough curves fell behind that of SMX, which was completely captured. Scarce literature exists for assessing biochar removal of pharmaceuticals and personal care products from WW in fixed-bed adsorbers. A few studies have quantified uptake of MPs from WW effluent by biochar using laboratory bench-scale column tests (Kimbell et al., 2017). However, these and other biochar studies suffer from significant limitations such as using adsorbate concentrations much higher than typically found in WW effluent or environmental waters. In addition, most studies use biochars generated under laboratory conditions such as furnaces purged with inert gases and subjected to chemical modification by washing with strong acids or bases, which has been shown to dramatically alter biochar adsorption properties (Zhang et al., 2013). Experiments using high adsorbate concentrations and laboratory produced biochars are unlikely to accurately simulate real-world treatment scenarios (Kearns et al., 2019). Finally, biochar-WW column studies have mostly not made use of mass transfer modeling approaches for downscaling mass transfer parameters from full-sized systems. A companion study to this article presented a detailed analysis of MP uptake from WW by biochar and GAC along with a mass transport modeling approach to predict MP breakthrough in full-scale biochar and GAC adsorbers from bench-scale column data (Kearns et al., 2020).
There are two studies that have quantified PFAS removal from WW by fixed-bed adsorbers. One of the studies simulated a WW treatment biofilter using a much lower hydraulic loading rate than typical for drinking water treatment system, and processed only 14 BV of test water over the course of the study (Dalahmeh et al., 2019). The other study performed WW pilot column tests using the same municipal WW and biochar (D-FD-HWP-900) and GAC as described in this study (Inyang and Dickenson, 2017). However, early PFAS breakthrough (i.e., BV10%) was not well captured in this study. So far, we are not aware of any studies to quantify biochar uptake of PFAS by landfill or open dump LEs. In LE experiments reported here, mass transfer zones were well captured for all PFAS in the GAC column and for all PFAS except PFBA and PFPeA in the D-FD-HWP-900 column (Fig. 1 and Supplementary Fig. S3). The GAC column test was discontinued at around 6,000 BV due to the limited volume of test water available. At this point, PFHpA, PFOA, PFNA, PFDA, and PFOS were below detection levels in GAC column effluent. Breakthrough curves for these compounds in the biochar column are presented in Supplementary Fig. S3.
Relative efficacy of adsorbents and effect of mode-of-contact
As a basis for comparison between adsorbates, adsorbents, and waters, this article considers the number of BV treated to 10% breakthrough of a given MP (i.e., C/C0 = 0.1), denoted BV10%. BV10% values estimated from breakthrough curves for all combinations of adsorbate, adsorbent, and water in this study are presented in Supplementary Data and Supplementary Tables S2 and S3. The relative efficacy for MP uptake by GAC and biochars was similar for this study as observed in previous work: GAC > high temperature CPTA biochars > moderate temperature CPTA biochars ≈ high-temperature CAP biochars (Kearns et al., 2019, 2020). Previous batch isotherm studies showed that high-temperature CPTA biochars can possess MP adsorption capacity comparable to ACs (Kearns et al., 2015b, 2019, Shimabuku et al., 2016). However, in this bench-scale column study, the GAC MP adsorption capacity exceeded the MP adsorption capacity of the representative CPTA biochar D-FD-HWP-900 by factors ranging between 2 and 34 (average 15). This range is similar to observations by Greiner et al. (2018) that fresh, regenerated, and heat-treated biochars had RSSCT adsorbent use rates ∼5–18 times that of GAC for uptake of SMX from SW. This discrepancy is partly explained by difference in the mode of contact. In batch-mode contactors, direct competition between target adsorbates and small molecular weight DOM moieties for adsorption sites within adsorbent pores reduces MP uptake capacity (Velten et al., 2011; Zietzschmann et al., 2014a, 2016a, 2016b; Kennedy and Summers, 2015; Shimabuku et al., 2017 ). Fixed-bed adsorbers are subject to time-dependent DOM preloading that makes both direct site competition as well as pore constriction and blockage by larger DOM molecules (“fouling”) important in determining adsorbent utilization rate (Velten et al., 2011; Corwin and Summers, 2012; Kennedy and Summers, 2015; Shimabuku et al., 2017). To assess the impact of multiple DOM fouling mechanisms on MP uptake from SW and WW by GAC and biochars, adsorbent use rates are compared for achieving 90% removal in batch mode with 10% breakthrough (i.e., 90% removal) in column mode. Batch mode use rates were determined by interpolating the dose of adsorbent required to achieve 90% MP removal (in mg/L) from dose–response curves. Batch study results were reported previously (Kearns et al., 2019, 2020). Column mode use rates were determined by dividing the adsorbent mass (in mg) by the volume of liquid passed through the column (in L) when BV10% was reached, as shown by Equation (2). Results of batch-column comparison are shown in Fig. 2 and tabulated in Supplementary Table S4. If 90% MP removal could be achieved with the same adsorbent use rate in either column or batch mode, data would be expected to fall along the 1:1 line shown in Fig. 2. The data shown in Fig. 2 underscore that caution should be applied when trying to project fixed-bed adsorber performance from batch adsorption data.
FIG. 2.
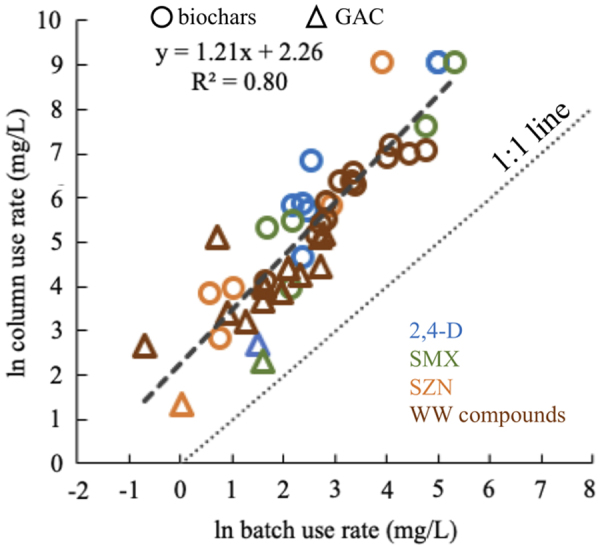
Relationship between adsorbent use rates for MP uptake in batch and fixed-bed contactors. MP, micropollutant.
An important practical implication of results discussed in this section is that, despite the typically lower cost of biochar compared to AC, in circumstances where AC is available, it may be the more cost-effective choice if the differential in adsorption capacity between biochar and AC exceeds the cost differential. This is in agreement with previous findings (Thompson et al., 2016). Also, biochar adsorbent use rates are closer to those of AC in batch-mode contactors than in fixed-bed adsorbers using granular media. Thus in some circumstances, powdered biochar contactors might be cost-competitive with powdered AC systems, whereas fixed-bed contactors using granular media would not.
Effect of particle size, EBCT, and DOM preloading on MP breakthrough
CD-RSSCT operational parameters for experiments used to determine the effects of particle size and contact time are provided in Supplementary Table S1. The effect of particle size on breakthrough of 2,4-D and SMX in SW experiments is illustrated by comparing data in Supplementary Fig. S1a, t, and u for D-FD-HWP-900 biochar, and Supplementary Fig. S1p, v, and w for GAC. As average biochar particle size decreased from 0.165 to 0.108–0.059 mm, 2,4-D BV10% values increased from 790 to 1,025–2,800 BV, respectively, and SMX BV10% values increased from 1,380 to 1,540–5,340 BV, respectively. As average GAC particle size decreased from 0.165 to 0.108–0.059 mm, 2,4-D BV10% values increased from 30,400 to 32,800–40,620 BV, respectively, and SMX BV10% values increased from 46,700 to 48,350–61,180 BV, respectively. These values are provided in Supplementary Table S2. It is expected that reducing adsorbent particle size can improve MP uptake because (1) diffusion pathlengths in smaller particles are shorter, which results in faster adsorption kinetics, and (2) crushing adsorbent to smaller particles exposes more of the interior, potentially opening up “blind pores” which cannot be accessed in larger particles. In SW experiments, reducing biochar particle size from 0.16 to 0.059 mm results in a 3.5 × increase in treatment capacity for 2,4-D and a 3.9 × increase in treatment capacity for SMX. Reducing GAC particle size from 0.165 to 0.059 mm resulted in a 1.3 × increase in treatment capacity for 2,4-and SMX. Thus, compared with GAC, the particle size effect appears to be stronger for biochar. As an adsorbent produced from a highly optimized industrial process, it is likely that GAC has better-developed pore interconnectivity than biochars produced from rudimentary pyrolysis technology. This has two important implications for biochar water treatment practitioners. The first is that, wherever possible, it is important to use as small of biochar particle size as can be obtained practically that will not result in excessive head-loss and clogging of the treatment unit. The use of upstream processes to remove particles, such as slow-sand filtration, could permit the use of smaller biochar particle sizes with less risk of clogging. The second implication is that it is likely to be a worthwhile effort to develop means of achieving pseudo-activation of biochars to promote pore interconnectivity and accessibility that can be implemented in low-resource scenarios. This is currently an area of active research.
Two methods were used to quantify the effect of increasing EBCT on uptake of 2,4-D and SMX by D-FD-HWP-900 biochar (Supplementary Fig. S1x, y). One method (“2xL”) increased the RSSCT bed depth from 10 to 20 mm, while holding other column operational parameters (i.e., flow rate, loading rate, particle size) constant. At the full-scale, this would be equivalent to adsorber A in Table 1 with a bed depth of 60 cm and an EBCT of 304 min. The other method (0.4xQ) reduced the RSSCT flow rate (Q) to 40% of the rate used in comparative experiments. This corresponds to a full-scale biochar contactor with the specifications listed for adsorber A in Table 1, but with a 380 min EBCT. Units designed in this manner produce 300 L of treated water per day and are widely used in villages throughout SE Asia (Kearns et al., 2016). Doubling the bed depth (i.e., the 2xL method) increased 2,4-D BV10% from 1,025 to 1,800 BV, and SMX BV10% from 1,540 to 3,120 BV. Slowing the flow rate (i.e., the 0.4xQ method) increased 2,4-D BV10% to 2,160 BV and SMX BV10% to 3,970 BV. These values are provided in Supplementary Table S2. RSSCT columns made from HPLC guard column hardware and fittings were not available for constructing a 2.5xL column to obtain an equivalent EBCT with the 0.4xQ method. However, extrapolating from the 2xL column experiments, results compare favorably with the 0.4xQ method. A 2.5xL column would be expected to increase 2,4-D BV10% to ∼2,190 BV (2,160 BV for 0.4xQ) and SMX BV10% to ∼3,910 BV (3,970 BV for 0.4xQ). Based on this study, increasing the EBCT of biochar contactors appears to benefit (i.e., reduce) the adsorbent use rate (the volume of water that can be treated per mass of adsorbent). This finding is corroborated by observations made by Greiner et al. (2018) who found that increasing EBCT from 10 to 30 min resulted in a decrease in adsorbent use rate. These findings are in contrast to some studies with GAC that showed adsorbent use rates to increase with increasing EBCT in the range of 5–30 minutes (Summers et al., 2011; Corwin and Summers, 2012; Kennedy et al., 2015). This phenomenon was attributed to increased fouling with longer service times, especially in the lower reaches of the GAC bed. In contrast, other studies with GAC used for treatment of landfill LE showed long (30–90 minutes) EBCTs to be optimal (Halim et al., 2010; Singh et al., 2012; Luo et al., 2020). The influence of EBCT on biochar uptake of MPs cannot be fully explained at this time. It can be speculated that MP diffusion into biochar pores is slow compared to GAC, and thus using long EBCTs benefits biochar adsorbent use rates.
The effect of preloaded DOM is illustrated in Supplementary Fig. S1z. Breakthrough curves for coloaded 2,4-D and SMX are shown with faint lines and symbols for visual reference. The D-FD-HWP-900 biochar RSSCT column was exposed to SW containing DOM at a concentration of 4 mg/L DOC for ∼50,000 BV before introduction of 2,4-D and SMX in the influent. This extent of DOM preloading nearly exhausted biochar capacity for uptake of MPs. Preloaded adsorbent breakthrough curves for 2,4-D and SMX quickly converge with breakthrough curves from the equivalent coloading experiment. In real-world treatment systems, 2,4-D, SMX, and other target MPs may be intermittently rather than continuously present. Preloading experiments presented here suggest that biochar bed life under intermittent MP loading regimes can be well-approximated by assuming continuous MP loading over the bed lifecycle.
The qualitative practical implication for biochar water treatment practitioners of the experiments discussed in this section is that, to the limits of practicality, long contact times and small particle sizes should be used.
Effect of DOM on adsorption and desorption
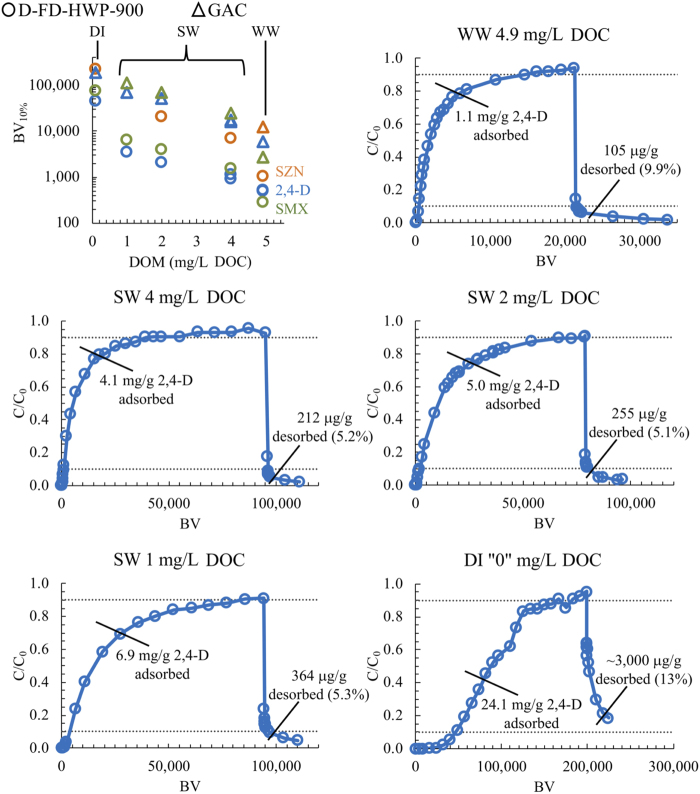
The data shown in Fig. 3 indicate that background DOM significantly reduced the MP adsorption capacity of GAC and biochar. In SW, 2,4-D adsorption capacity of D-FD-HWP-900 biochar declined from 6.9 to 4.1 mg/g at BV10% as DOM concentration increased from 1 to 4 mg/L DOC. WW DOM had a proportionally greater effect than SW on reduction of MP adsorption capacity on a concentration basis, in agreement with other studies (Velten et al., 2011; Zietzschmann et al., 2016b; Sgroi et al., 2018). This is likely due to the greater prevalence of low-molecular-weight acidic and neutral constituents in WW DOM compared with SW DOM, as these constituents are most responsible for adsorbent fouling (Velten et al., 2011; Zietzschmann et al., 2016b; Sgroi et al., 2018). As expected, much higher adsorption capacity was observed in laboratory clean “DI” water. This underscores the importance of conducting adsorption studies in properly matched background matrices.
FIG. 3.
Effect of DOM concentration on MP adsorption and desorption. DOM, dissolved organic matter.
MP desorption was investigated for biochars D-FD-HWP-900, C-FD-PINE-875, C-FD-PEC-900, C-FD-CHER-875, D-ND-EUC-850, C-ND-PINE-625, C-ND-CHER-650, and W-R-600-3d (S1). Representative 2,4-D adsorption and desorption data from D-FD-HWP-900 biochar in WW, SW, and DI water experiments are shown in Fig. 3. 2,4-D desorption expressed as a mass percentage of 2,4-D adsorbed during the loading phase of the experiment was small: <10% in WW and around 5% in SW. Similar desorption trends were observed for SMX and SZN in SW and WW (data not shown). Greater MP desorption was observed in DI water experiments. This suggests that desorption of adsorbed MPs is hindered when DOM is present. This could be explained by incoming DOM restricting pore throats and inhibiting the back-diffusion of adsorbed MPs during column operation, as has been observed for GAC (Corwin and Summers, 2011). DOM constituents themselves have been shown to adsorb irreversibly (Velten et al., 2011; Kennedy and Summers, 2015), supporting the hypothesis of DOM-hindered MP back-diffusion. In contrast, LE experiments did indicate desorption of some PFAS (i.e., C/C0 >1.0) from biochar and GAC despite the presence of DOM, presumably through displacement of weakly adsorbing compounds by more strongly adsorbing DOM constituents and/or PFAS moieties (Supplementary Fig. S3). In the biochar LE experiment, significant desorption was observed for PFBA, PFPeA, PFHxA, PFHpA, and PFOA. PFBA and PFPeA were not well removed by the D-FD-HWP-900 biochar. With the exception PFBA and PFPeA, desorption of the other PFAS from biochar occurred after around 1,500 BV, whereas BV10% values for the other PFAS ranged from 460 (PFHpA) to 1,500 (PFOS) (Supplementary Fig. S3; Supplementary Table S3). Thus, from this experiment, it appears that if a biochar treatment system was managed for high levels of removal of most PFAS, biochar regeneration or replacement would happen before substantial desorption would be expected to occur.
A practical implication of the experimental results discussed in this section for biochar water treatment is that DOM significantly reduces biochar capacity for MP uptake. Where possible, upstream treatment steps to reduce DOM in the influent to biochar adsorbers such as coagulation (e.g., using Moringa oleifera) or biofiltration (e.g., using slow-sand biofiltration) should be implemented to extend biochar bed life for removal of weakly adsorbing MPs.
UVA254 as a surrogate for adsorbent bed life
Figure 1 and Supplementary Fig. S1–S3 display UVA254 breakthrough data collected simultaneously with MP breakthrough data. UVA254 breakthrough preceded MP breakthrough for all experimental conditions in this study. To investigate the potential use of UVA254 breakthrough as a surrogate for adsorbent bed life, UVA254 relative concentrations (C/C0) were estimated at corresponding MP BV10% values. In SW, 2,4-D was the first MP to break through. The corresponding UVA254 C/C0 values ranged from 0.83 to 0.95 with an average of 0.90 for CPTA biochars, and ranged from 0.92 to 0.98 with an average of 0.95 or GAC. For SMX, the second compound to break through in SW, the corresponding UVA254 C/C0 values were all ≥0.90 for CPTA biochars. This result agrees favorably with observations made by Greiner et al. (2018) in RSSCT studies of SMX uptake from SW by fresh and regenerated biochars.
In WW, SMX was the first MP to break through. Its corresponding UVA254 C/C0 values were 0.67 for D-FD-WHP-900 biochar and 0.70 for GAC. In LE, D-FD-HEP-900 biochar column data were insufficient for estimating UVA254 C/C0 values that correspond to BV10% of PFBA and PFPeA, the first two compounds to break through. For PFBS, the third compound to break through, the corresponding UVA254 C/C0 value was 0.80. All other PFAS studied had UVA254 C/C0 values at or above 0.87 in the biochar column. For the GAC column, UVA254 C/C0 values that correspond to BV10% of PFBA and PFPeA were 0.62 and 0.78, respectively. For the MP-water-adsorbent combinations studied here, no single UVA254 C/C0 value corresponded to early breakthrough of the most weakly adsorbing MPs independent of water type. However, in these column tests, a UVA254 C/C0 value of 0.60 indicated ≥90% removal of all MPs by GAC and all MPs except PFBA and PFPeA by biochar. Although this study did not examine the same MPs in LE and SW/WW, a comparison can be drawn based on other work that demonstrated that early SMX breakthrough falls significantly after PFOA in SW and WW (Anumol et al., 2015; Sgroi et al., 2018), and PFOA was one of the strongest adsorbed PFAS among the compounds studied here. Therefore, it is unlikely that 2,4-D or SMX, the first compounds to break through in SW and WW columns, would have also been the first to break through LE columns.
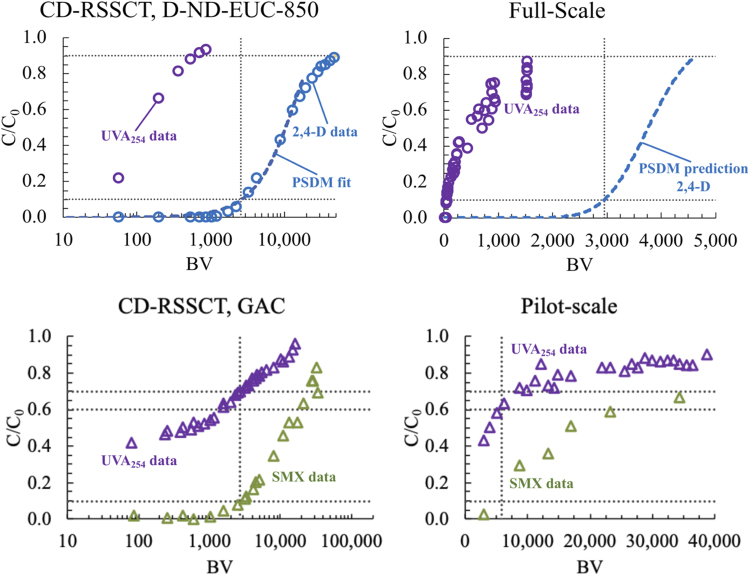
In this study, UVA254 and MP breakthrough relationships were obtained from RSSCT studies in the laboratory. As a preliminary effort to test the scalability of UVA254 as a conservative surrogate for bed life under field conditions, UVA254 breakthrough data were obtained from pilot-scale and full-scale adsorbers. The full-scale system, which has been operated by a farming community in northern Thailand treating SW since 2008 and has been through several biochar replacement cycles, has a bed depth of 1.25 m, an EBCT of around 18 h, and an average particle diameter of 0.75 cm. The parameters governing mass transfer in this system are similar to those of the systems described in Table 1 for full-scale adsorber and RSSCT design A. The upper-left panel of Fig. 4 shows RSSCT data for UVA254 and 2,4-D breakthrough in SW with 4 mg/L DOC for the D-ND-EUC-850 biochar—the same biochar used in the full-scale system. 2,4-D data were not available for the full-scale system, so a modeling approach was applied to simulate a 2,4-D breakthrough curve. The pore and surface diffusion model was fitted to 2,4-D RSSCT data and used to predict 2,4-D removal at full scale using the procedure described by Summers et al. (2014) and the AdDesignS software package (Michigan Technological University, Houghton, MI). The predicted 2,4-D breakthrough curve is shown in the upper-right panel of Fig. 4. 2,4-D physical-chemical properties inputted into AdDesignS are provided in Supplementary Table S5. Full-scale UVA254 data collected over >1,500 BV, corresponding to one adsorbent replacement cycle, indicate that using a UVA254 C/C0 value of around 0.85 (±0.05) would likely provide a rough conservative surrogate for achieving high levels of 2,4-D removal in this system.
FIG. 4.
Relationships between UVA254 breakthrough and 2,4-D breakthrough for biochar treating SW, and between UVA254 breakthrough and SMX breakthrough for GAC treating WW at small column (left panels) and large column (right panels) scales. In the upper panels, the pore and surface diffusion model was fitted to CD-RSSCT data (left) and used to predict 2,4-D breakthrough in a full-scale biochar water treatment system (right). In the lower panels, UVA254 and SMX breakthrough were directly compared in the CD-RSSCT (left) and pilot column (right). 2,4-D, 2,4-dichlorophenoxyacetic acid; SMX, sulfamethoxazole; RSSCT, rapid small-scale column test; CD, constant diffusivity.
The pilot-scale column containing the same GAC and treating the same WW used in RSSCT experiments had an EBCT of 7.9 min, a bed depth of 2.9 m, and an average particle diameter of 1.29 mm (corresponding to 8 × 30 United States Standard mesh sieve sizes). The parameters governing mass transfer in this system are similar to those of the systems described in Table 1 for full-scale adsorber and RSSCT design A. The data in the lower left panel in Fig. 4 show that in the RSSCT experiment UVA254 C/C0 was equal to 0.65–0.70 at SMX BV10%. The data in the lower right panel indicate that in the pilot column UVA254 C/C0 was equal around 0.60 at SMX BV10%, which is similar to the RSSCT. However, SMX pilot column data are insufficient to resolve SMX early breakthrough with high precision—thus these results should be considered tentative and explored further.
Using absolute and relative UVA254 to estimate biochar bed lifecycle
Results of this study suggest that UVA254 relative breakthrough is a promising conservative surrogate for adsorbent bed life. One way that the study results could be applied is to consider weakly adsorbing sentinel MPs paired with relevant background water matrices such as short-chain PFAS in LE, SMX in WW, and 2,4-D in SW. Relative UVA254 breakthrough values (C/C0) of ∼0.6, 0.7, and 0.9, for these water-MP combinations, respectively, correspond with high levels of MP control (∼90% removal of weakly adsorbing sentinel compounds, >90% removal of the more strongly adsorbing compounds) for the MP-water combinations included in the study. Because UVA254 breakthrough curves flatten with service time (e.g., due to biodegradation of some UV absorbing DOM constituents in the adsorbent bed and slow adsorption of larger molecular weight DOM fractions), UVA254 C/C0 bed life indicator values >90% should be avoided. Also, because many UV absorbing DOM constituents break through early in the adsorbent bed lifecycle, selecting UVA254 C/C0 indicator values <60% would probably be impractical as it would lead to very short service times (e.g., 100–200 BV under the study conditions). The absolute UVA254 values for SW, WW, and LE were 0.128, 0.234, and 0.411 cm−1, respectively. A plot of absolute UVA254 values versus respective relative breakthrough values indicating high levels of MP control is shown in Fig. 5, and fitted with the power law shown in Equation (3). The absolute UVA254 value of a water reflects both the total DOM concentration and its spectrochemical character (i.e., aromaticity), parameters that simultaneously influence adsorbent fouling. Thus, the absolute UVA254 value of a water source could be inputted in to provide an approximate guide for selecting a relative UVA254 breakthrough value to indicate the need for biochar regeneration or replacement. These results are based on experiments with only three waters and should be further investigated. Operator caution, periodic MP monitoring for validation, and appropriate safety factor(s) should be applied. However, this approach opens up new possibilities for GAC and biochar water treatment system monitoring in economically and resource constrained settings.
FIG. 5.
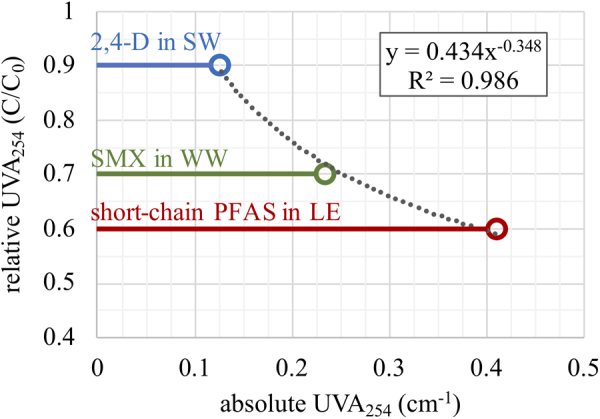
Relationship between absolute UVA254 values of SW, WW, and LE and relative UVA254 breakthrough values that indicate a likelihood of high levels of MP removal in biochar fixed-bed adsorbers.
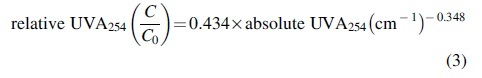
A description of the necessary field equipment and supplies along with a step-by-step guide to measuring UVA254 and estimating biochar bed lifecycle in the field is provided in Supplementary Data.
Conclusions
This study quantified removal of weakly and moderately adsorbing organic MPs from SW, WW, and LE by laboratory bench-scale fixed-bed adsorbers containing biochar and GAC. More attention was given to weakly adsorbing MPs as these would likely dictate adsorbent replacement frequency. While many studies have quantified biochar adsorption of MPs in batch mode contactors, comparatively very few studies have utilized mass transfer model-based scaling approaches to simulate full-scale fixed-bed biochar adsorbers. Some studies have shown biochars produced under high temperature CPTA conditions to exhibit MP adsorption capacity comparable to AC in batch tests where direct site competition is the primary DOM fouling mechanism. However, biochar adsorbent use rates can be significantly higher than those of GAC in column mode contactors. For some applications, this use rate tradeoff could more than offset the typically lower cost on a per-mass basis of biochar compared with GAC. However, in many applications, in particular in low-resource settings such as the developing world, GAC is often unobtainable due to cost or supply-chain limitations, whereas biochar can be generated on-site using “low tech” methods and local surplus biomass.
In qualitative terms, optimizing biochar water treatment systems for MP control calls for using a relatively uniform distribution of as small of particle sizes as can be obtained using locally available equipment while avoiding head loss and clogging of the adsorbent column by biochar fines. It also calls for using long EBCTs (30 min to several hours) to the limits of practicality and cost for the size of the contactor relative to the necessary throughput. Particle size and contact time scale with one-another, so using larger particle sizes can be partly compensated by designing for longer EBCTs, and vice versa. Removal of MPs in biochar adsorbers benefits from upstream treatment processes such as slow-sand biofiltration to remove particulates and reduce the impact of DOM fouling. Selecting the biochar regeneration/replacement frequency based on weakly adsorbing “sentinel” chemical MPs and some knowledge of background water characteristics (e.g., SW impacted by WW, etc.) presents two useful advantages. One is that the potential for displacement and desorption of contaminants of concern by more strongly adsorbing MPs or DOM fractions resulting in elevated concentrations in biochar contactor effluent is minimized. The second is that monitoring biochar bed life using the relative removal of UVA254-DOM can provide a rough conservative indicator for high levels of MP removal. With operator judgment and consideration of source water properties, UVA254 C/C0 values in the range of 0.6–0.9 can be selected to signal the end of a biochar bed lifecycle. This opens up new possibilities for monitoring the control of MPs in treatment systems using carbonaceous adsorbents when quantifying individual trace chemicals of concern is out-of-reach due to logistical, analytical, and/or cost barriers.
Supplementary Material
Acknowledgments
The authors give special thanks to the Southern Nevada Water Authority's staff, including Mandu Inyang and Marco Velarde, for performing the pilot-scale column testing of wastewater in Las Vegas with GAC, Oscar Quiñones for the analysis of native micropollutants in the wastewater from Las Vegas, Brett Vanderford for data review, and Janie Holady and Josephine Chu for sample preparation, coordination, and UVA254 analysis.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
US EPA Science to Achieve Results (STAR) graduate research fellowship Southern Nevada Water Authority, North Carolina State University and University of Sri Jayewardenepura (Sri Lanka) Partnership for Excellence in Global WaSH (Water-Sanitation-Hygiene) Research Aqueous Solutions.
Supplementary Material
References
- Anumol, T., Sgroi, M., Park, M., Roccaro, P., and Snyder, S.A. (2015). Predicting trace organic compound breakthrough in granular activated carbon using fluorescence and UV absorbance as surrogates. Water Res. 76, 76. [DOI] [PubMed] [Google Scholar]
- Corwin, C.J., and Summers, R.S. (2011). Adsorption and desorption of trace organic contaminants from granular activated carbon adsorbers after intermittent loading and throughout backwash cycles. Water Res. 45, 417. [DOI] [PubMed] [Google Scholar]
- Corwin, C.J., and Summers, R.S. (2012). Controlling trace organic contaminants with GAC adsorption. J. Am. Water Works Assoc. 104, 43 [Google Scholar]
- Dalahmeh, S.S., Alziq, N., and Ahrens, L. (2019). Potential of biochar filters for onsite wastewater treatment: Effects of active and inactive biofilms on adsorption of per- and polyfluoroalkyl substances in laboratory column experiments. Environ. Pollut. 247, 155. [DOI] [PubMed] [Google Scholar]
- Greiner, B.G., Shimabuku, K.K., and Summers, R.S. (2018). Influence of biochar thermal regeneration on sulfamethoxazole and dissolved organic matter adsorption. Environ. Sci. Water Res. Technol. 4, 169 [Google Scholar]
- Halim, A.A., Aziz, H.A., Johari, M.A.M., Ariffin, K.S., and Adlan, M.N. (2010). Ammoniacal nitrogen and COD removal from semi-aerobic landfill leachate using a composite adsorbent: Fixed bed column adsorption performance. J. Hazard. Mater. 175, 960. [DOI] [PubMed] [Google Scholar]
- Inyang, M., and Dickenson, E.R.V. (2017). The use of carbon adsorbents for the removal of perfluoroalkyl acids from potable reuse systems. Chemosphere 184, 168. [DOI] [PubMed] [Google Scholar]
- Kearns, J., Dickenson, E., and Knappe, D. (2020). Enabling organic micropollutant removal from water by full-scale biochar and activated carbon adsorbers using predictions from bench-scale column data. Environ. Engi. Sci. 37, 459 [Google Scholar]
- Kearns, J.P., Knappe, D.R.U., and Summers, R.S. (2014). Synthetic organic water contaminants in developing communities: An overlooked challenge addressed by adsorption with locally generated char. J. Water Sanit. Hyg. Dev. 4, 422 [Google Scholar]
- Kearns, J.P., Knappe, D.R.U., and Summers, R.S. (2015a). Feasibility of using traditional kiln charcoals in low cost water treatment: The role of pyrolysis conditions on 2,4-D herbicide adsorption. Environ. Eng. Sci. 32, 912 [Google Scholar]
- Kearns, J.P., Reents, N., and Deriemaeker, B. (2016). 300 Liter per Day Biochar Water Treatment System Graphical Manual. Chiang Mai, Thailand: Aqueous Solutions [Google Scholar]
- Kearns, J.P., Shimabuku, K.K., Knappe, D.R.U., and Summers, R.S. (2019). High temperature co-pyrolysis thermal air activation enhances biochar adsorption of herbicides from surface water. Environ. Eng. Sci. 36, 7103 [Google Scholar]
- Kearns, J.P., Shimabuku, K.K., Mahoney, R.B., Knappe, D.R.U., and Summers, R.S. (2015b). Meeting multiple water quality objectives through treatment using locally generated char: Improving organoleptic properties and removing synthetic organic contaminants and disinfection by-products. J. Water Sanit. Hyg. Dev. 5, 359 [Google Scholar]
- Kennedy, A.M., Reinert, A.M., Knappe, D.R.U., Ferrer, I., and Summers, R.S. (2015). Full- and pilot-scale GAC adsorption of organic micropollutants. Water Res. 68, 238. [DOI] [PubMed] [Google Scholar]
- Kennedy, A.M., Reinert, A,M., Knappe, D.R.U., and Summers, R.S. (2017). Prediction of full-scale GAC adsorption of organic micropollutants. Environ. Eng. Sci. 34, 496 [Google Scholar]
- Kennedy, A.M., and Summers, R.S. (2015). Effect of DOM size on organic micropollutant adsorption by GAC. Environ. Sci. Technol. 49, 6617. [DOI] [PubMed] [Google Scholar]
- Kimbell, L.K., Tong, Y., Mayer, B.K.., and McNamara, P.J. (2017). Biosolids-derived biochar for triclosan removal from wastewater. Environ. Eng. Sci. 35, 513 [Google Scholar]
- Knappe, D.R.U., Snoeyink, V.L., Roche, P., Prados, M.J., and Bourbigot, M.M. (1999). Atrazine removal by preloaded GAC. J. Am. Water Works Assoc. 91, 97 [Google Scholar]
- Korak, J.A., Dotson, A.D., Summers, R.S., and Rosario-Ortiz, F.L. (2014). Critical analysis of commonly used fluorescence metrics to characterize dissolved organic matter. Water Res. 49, 327. [DOI] [PubMed] [Google Scholar]
- Li, Q., Snoeyink, V.L., Mariãas, B.J., and Campos, C. (2003b). Elucidating competitive adsorption mechanisms of atrazine and NOM using model compounds. Water Res. 37, 773. [DOI] [PubMed] [Google Scholar]
- Li, Q.L., Snoeyink, V.L., Marinas, B.J., and Campos, C. (2003a). Pore blockage effect of NOM on atrazine adsorption kinetics of PAC: The roles of PAC pore size distribution and NOM molecular weight. Water Res. 37, 4863. [DOI] [PubMed] [Google Scholar]
- Luo, H., Zeng, Y., Cheng, Y., He, D., and Pan, X. (2020). Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 703, 135468. [DOI] [PubMed] [Google Scholar]
- Sgroi, M., Anumol, T., Roccaro, P., Vagliasindi, F.G.A., and Snyder, S.A. (2018). Modeling emerging contaminants breakthrough in packed bed adsorption columns by UV absorbance and fluorescing components of dissolved organic matter. Water Res. 145, 667. [DOI] [PubMed] [Google Scholar]
- Shimabuku, K.K., Cho, H., Townsend, E.B., Rosario-Ortiz, F.L., and Summers, R.S. (2014). Modeling nonequilibrium adsorption of MIB and sulfamethoxazole by powdered activated carbon and the role of dissolved organic matter competition. Environ. Sci. Technol. 48, 13735. [DOI] [PubMed] [Google Scholar]
- Shimabuku, K.K., Kearns, J.P., Martinez, J., Mahoney, R.B., Moreno-Vasquez, L., and Summers, R.S. (2016). Biochar sorbents for sulfamethoxazole removal from surface water, stormwater, and wastewater effluent. Water Res. 96, 236. [DOI] [PubMed] [Google Scholar]
- Shimabuku, K.K., Kennedy, A.M., Mulhern, R.E., and Summers, R.S. (2017). Evaluating activated carbon adsorption of dissolved organic matter and micropollutants using fluorescence spectroscopy. Environ. Sci. Technol. 51, 2676. [DOI] [PubMed] [Google Scholar]
- Singh, S.K., Townsend, T.G., Mazyck, D., and Boyer, T.H. (2012). Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res. 46, 491. [DOI] [PubMed] [Google Scholar]
- Summers, R.S., Kennedy, A.M., Knappe, D.R.U., Reinert, A.M., Fotta, M.E., Mastropole, A.J., Corwin, C.J., and Roccaro, J. (2014). Evaluation of Available Scale-Up Approaches for the Design of GAC Contactors. Denver, CO: Water Research Foundation [Google Scholar]
- Summers, R.S., Knappe, D.R.U., and Snoeyink, V.L. (2011). Adsorption of organic compounds by activated carbon. In J.K. Edzwald, Ed., Water Quality and Treatment: A Handbook on Drinking Water. Denver, CO: American Water Works Association, p. 1117 [Google Scholar]
- Thompson, K.A., Shimabuku, K.K., Kearns, J.P., Knappe, D.R.U., Summers, R.S., and Cook, S.M. (2016). Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 50, 11253. [DOI] [PubMed] [Google Scholar]
- Velten, S., Knappe, D.R.U., Traber, J., Kaiser, H.P., von Gunten, U., Boller, M., and Meylan, S. (2011). Characterization of natural organic matter adsorption in granular activated carbon adsorbers. Water Res. 45, 3951. [DOI] [PubMed] [Google Scholar]
- Weishaar, J.L., Aiken, G.R., Bergamaschi, B.A., Fram, M.S., Fujii, R., and Mopper, K. (2003). Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 37, 4702. [DOI] [PubMed] [Google Scholar]
- Zhang, P., Sun, H.W., Yu, L., and Sun, T.H. (2013). Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biocharse. J. Hazard. Mater. 244, 217. [DOI] [PubMed] [Google Scholar]
- Zietzschmann, F., Altmann, J., Ruhl, A.S., Dünnbier, U., Dommisch, I., Sperlich, A., Meinel, F., and Jekel, M. (2014b). Estimating organic micro-pollutant removal potential of activated carbons using UV absorption and carbon characteristics. Water Res. 56, 48. [DOI] [PubMed] [Google Scholar]
- Zietzschmann, F., Aschermann, G., and Jekel, M. (2016a). Comparing and modeling organic micro-pollutant adsorption onto powdered activated carbon in different drinking waters and WWTP effluents. Water Res. 102, 190. [DOI] [PubMed] [Google Scholar]
- Zietzschmann, F., Müller, J., Sperlich, A., Ruhl, A.S., Meinel, F., Altmann, J., and Jekel, M. (2014a). Rapid small-scale column testing of granular activated carbon for organic micro-pollutant removal in treated domestic wastewater. Water Sci. Technol. 70, 1271. [DOI] [PubMed] [Google Scholar]
- Zietzschmann, F., Stützer, C., and Jekel, M. (2016b). Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater-aligning breakthrough curves and capacities. Water Res. 92, 180. [DOI] [PubMed] [Google Scholar]
- Ziska, A.D., Park, M., Anumol, T., and Snyder, S.A. (2016). Predicting trace organic compound attenuation with spectroscopic parameters in powdered activated carbon processes. Chemosphere 156, 163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.