Abstract
Background:
Transradial access (TRA) is associated with improved survival and reduced vascular complications in acute myocardial infarction (AMI). Limited data exist regarding TRA utilization and outcomes for AMI complicated by cardiogenic shock (CS). We sought to assess the safety, feasibility, and clinical outcomes of TRA in AMI-CS.
Methods:
One-hundred and fifty-three patients with AMI-CS were stratified into tertiles of disease severity using the CardShock score. The primary endpoint was successful percutaneous coronary intervention (PCI), defined as Thrombolysis in Myocardial Infarction III flow with survival to 30 days.
Results:
Mean age was 66 years, 72% were men, and 47% had diabetes. TRA was the preferred access site in patients with low and intermediate disease severity. Overall, 50 (32%) patients experienced major adverse cardiac and cerebrovascular events; most events (78%) occurred in patients undergoing transfemoral access (TFA) in the intermediate-high tertiles of CS severity. Of the 41 (27%) total bleeding events, 32% occurred at the coronary angiography access site, of which 92% were in the TFA group. The use of ultrasound (US) guidance for TFA resulted in reduced coronary access-site bleeding (8.5 vs. 33.0%, p = .01). In a hierarchical logistic regression model, utilizing TRA did not result in lower odds of successful PCI (Odds ratio [OR]: 1.36; 95% confidence interval [CI]: 0.54–3.40).
Conclusion:
This study suggests that TRA is feasible across the entire spectrum of AMI-CS and is associated with reduced coronary access-site bleeding. In addition, US-guided TFA is associated with reductions in access-site bleeding and vascular complications. Concerted efforts should be made to incorporate vascular access protocols into existing CS algorithms in dedicated shock care centers.
Keywords: cardiogenic shock, mechanical circulatory support, percutaneous coronary intervention, transradial coronary angiography
1 |. INTRODUCTION
Despite advances in early revascularization, regionalized systems of ST-elevation myocardial infarction (STEMI) care, and the advent of mechanical circulatory support (MCS) devices, clinical outcomes in acute myocardial infarction (AMI) complicated by cardiogenic shock (CS) remain poor, with mortality rates exceeding 40%.1–3 In addition to recurrent myocardial ischemia and multiorgan failure, patients with AMI-CS are at increased risk for bleeding, a powerful predictor of mortality in acute coronary syndromes.4 The increased bleeding risk is multifactorial and due to microcirculatory derangements, end organ hypoperfusion with resultant coagulopathy, adjuvant antithrombotic pharmacotherapy, and access-site-related complications.5
Transradial access (TRA) in AMI is associated with significant reductions in mortality, major bleeding, and vascular complications, when compared to transfemoral access (TFA).6 As TRA utilization continues to rise nationwide, it is now advocated as the default access site for coronary angiography and intervention.6,7 The clinical benefits of TRA in AMI-CS are less certain; however, these patients have either been underrepresented or excluded from randomized trials.8 Observational registries have demonstrated safety, feasibility, and mortality reductions with TRA in AMI-CS.9,10 In the absence of the uniform uptake in standardized disease severity definitions, gaps in knowledge exist regarding potential for selection bias in access-site utilization, especially in CS patients who present with a wide spectrum of disease severity.11 In addition, AMI-CS has been identified as an independent predictor of TRA failure, as these patients are often vasoconstricted and on temporizing vasopressors, and thus may have diminished radial pulses.12 Lastly, increasing number of AMI-CS patients are treated with temporizing MCS, which necessitates large bore vascular access, thereby increasing risk for bleeding events and transfusions.5,13 The primary aim of this study was to assess the safety, feasibility, patterns of use, and clinical outcomes of TRA across the spectrum of AMI-CS at a high-volume shock program utilizing standardized management protocols. We also sought to examine the clinical outcomes based on coronary angiography access-site utilization, stratified by degree of CS severity to account for baseline selection biases, which may predispose operators to favor TFA in more severe forms of AMI-CS.
2 |. METHODS
2.1 |. Study population
This retrospective cohort study included 153 consecutive patients presenting to a high-volume shock center with a diagnosis of AMI-CS, who underwent emergent coronary angiography with intent of early revascularization, from January 3, 2017 to June 30, 2019 (Figure 1). Our standardized CS treatment algorithm has been previously published.1 Patients were diagnosed with AMI (STEMI and non-STEMI) based on established clinical, electrocardiographic, and biochemical criteria.14 Diagnosis of CS was based on clinical and hemodynamic criteria as previously published in the should we emergently revascularize occluded coronaries for cardiogenic shock (SHOCK) trial.15 All patients were recommended to undergo right heart catheterization for comprehensive hemodynamic assessment. In consultation with the multidisciplinary CS team and based on clinical findings, patients were treated with primary percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) surgery, or medical therapy alone. We excluded patients who were not eligible for coronary angiography (e.g., concomitant hemorrhagic shock; inability to provide informed consent; patient and family wishes) and patients with acute decompensated heart failure.
FIGURE 1.
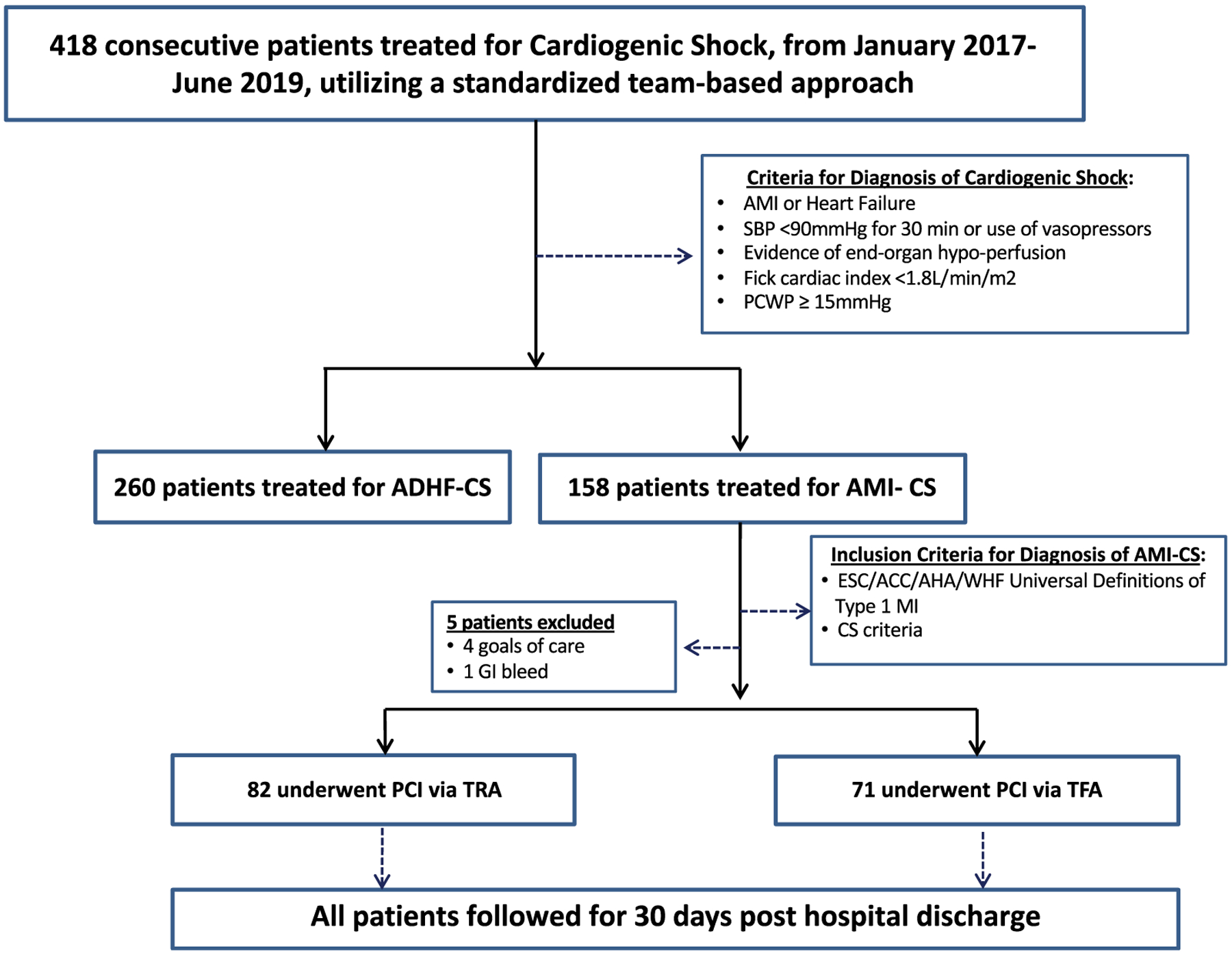
Study flow diagram
2.2 |. Coronary angiography and PCI for CS-AMI
Coronary angiograms were performed by experienced interventionalists who had performed at least 50 prior TRA PCIs.16 Access-site selection for coronary angiography was at the discretion of the operator. In patients undergoing TRA, access technique (single- or double-wall puncture), utilization of ultrasound (US), and intra-arterial vasodilators were left to clinical judgment. In accordance with institutional policy and contemporary best practices, all operators performing TFA were advised to use US and fluoroscopic guided micropuncture technique with follow-on selective femoral angiography prior to coronary angiography and intervention.17,18 In TFA patients requiring MCS for refractory CS, the contralateral femoral artery was primarily utilized. In TRA patients, postprocedure hemostasis was performed using a vascular compression device for 120 min after diagnostic catheterization or 240 min following PCI. In patients undergoing TFA for coronary angiography or MCS, hemostasis was obtained primarily using either Proglide (Abbott Inc., Chicago, IL) suture-based closure with or without balloon-occlusion “dry closure,” or manual compression. In patients with a percutaneous ventricular assist device, a final ipsilateral femoral run-off angiogram was recommended. If any evidence of compromised limb perfusion was noted, an ipsilateral antegrade distal perfusion catheter was considered.
2.3 |. Risk stratification
All patients in our registry were retrospectively assigned two clinical risk scores at the time of index CS diagnosis, for 30 day mortality: (a) The CardShock score which has been externally validated and includes seven variables predictive of short-term prognosis: age > 75 years, confusion, prior revascularization, ACS etiology, left ventricular ejection fraction <40%, baseline lactate, and renal function; and (b) The Society for Cardiovascular Angiography and Intervention (SCAI) CS classification system, which stratifies patients into five stages (A–E) based on an amalgamation of physical exam findings, biomarkers, and invasive hemodynamics.11,19 The SCAI CS nomenclature has been retrospectively validated for 30-day outcomes.20
2.4 |. Clinical outcomes
The primary endpoint of the study was successful PCI, defined as Thrombolysis in Myocardial Infarction (TIMI) III flow post-PCI combined with survival to 30 days following discharge. Secondary endpoints included major adverse cardiac and cerebrovascular events (MACCE), a composite of in-hospital mortality, re-infarction, target vessel revascularization, and stroke. Other secondary endpoints included major coronary and secondary vascular site access-site-related bleeding, defined as Bleeding Academic Research Consortium (BARC) types 3a, 3b, and 5 bleeds, nonvascular access-site bleeding (BARC 3c), and major vascular complications.21
2.5 |. Statistical analysis
To address potential selection bias associated with coronary access-site utilization in AMI-CS, baseline demographics, clinical characteristics, and hemodynamic data were compared in patients presenting with AMI-CS by severity of their illness at time of index CS diagnosis captured by tertiles of the CardShock risk score. Selection bias in the choice of coronary access site arises from the absence of comparability as the exposed and the unexposed (i.e., TRA vs. TFA) differ to an important degree by the severity of CS. For example, operators often tend to choose TFA in sicker patients with advanced degrees of CS, while in patients with lesser severity CS, TRA is more often utilized. Within each tertile of CS, a sub-classification by coronary access site was performed to understand the association between the choices of vascular access as it relates to the degree of CS. We also presented the baseline demographics, choice of vascular access, hemodynamics, and interventional characteristics according to the SCAI staging system at the time of index CS diagnosis. The data were presented as median (25th and 75th percentile), or frequency and percent. Comparisons were made using chi-square analysis, Fisher’s exact test, or Wilcoxon Rank Sum test, where appropriate. In similar fashion, angiographic and interventional characteristics were presented for each coronary access site by tertiles of the CardShock Score. We plotted the clinical outcomes among AMI-CS patients by coronary access site, including 30-day mortality, access-site bleeding, vascular complications, and MACCE.
To determine predictors of successful PCI, we used a 3-step hierarchal logistic regression approach to adjust for baseline characteristics (model 1), case severity (model 2), and access site (model 3) as proposed by Wong and Mason.22 Hierarchal multilevel logistic regression model assumes that there is variation at each level (i.e., patient characteristics [i.e., individual level], CS severity, and choice of coronary access site for angiography and intervention). Stated differently, the model assumes that each patient has an underlying, unobservable probability of TIMI III flow post-PCI combined with survival at 30 days, and that probability varies across levels of CS and choice of coronary access site. This model assumed a normal distribution in the degree of CS and choice of access-site levels. The proportion in the residual variance between each of three levels of hierarchy was determined for the outcome, using methods described by Wong and Mason. This is equal to the proportion of the unexplained variability in TIMI III flow and survival at 30 days due to unmeasured characteristics at each of the three levels.
The models were fitted using SAS (ver. 9.4, Cary, NC). At the first stage, the usual regression model was applied with the outcome being successful TIMI III flow post-PCI combined with survival at 30 days. The independent variables at the first stage included age at presentation (in 5-year categories), gender, body mass index (BMI), baseline systolic blood pressures, number of vasopressors prior to angiography, cardiac arrest, intubation, lactate at baseline, prior history of CABG, and use of MCS. These characteristics were deemed important for AMI-CS patients and were included based on historical relevance. In this model, we were able to account for individual level characteristics as it relates to TIMI III flow at end of the procedure, which led to survival at 30 days. At the second level, tertiles of Cardshock score were used in the multilevel logistic regression model to account for the variability in the degree of CS while the choice of vascular access was used as the third level in the model. A random effect of the CardShock risk score was included in the hierarchical model to take into account the cluster structure of the data. To test for unexplained variation in TIMI III flow and survival at 30 days between different risk groups, we fitted an empty model for the mean of the outcome (i.e., Successful TIMI III flow post-PCI and survival at 30 days) without an independent variable. To compare our multilevel model to the traditional logistic regression model, we restricted our analysis to only those variables that converged in both methods. Odds ratios (ORs) and 95% confidence intervals (CIs) were presented at the hierarchy for each of the three levels. p-values <.05, two sided, were considered statistically significant. The institutional review board at the INOVA Heart and Vascular Institute approved this study.
3 |. RESULTS
3.1 |. Patient characteristics
A total of 153 AMI patients presented with diagnosis of AMI-CS over the 18-month study period (Table 1). Median age was 66 years, 72% were males, and 47% had diabetes mellitus. Fifty-two percentage were transferred from outside institutions and 80% were receiving vasopressor therapy at time of diagnosis. At baseline, 50% of patients were supported with intra-aortic balloon pump (IABP) and 43% went on to receive axial or centrifugal-flow MCS devices. CS threshold markers at 24 hr were lactate <3.0 mg/dL [n (%) = 102 (67)], cardiac power output >0.6 W [n (%) = 88 (58)], and PAPi >1.0 [n (%) = 132 (86)]. When stratified by coronary access-site choice, the distribution of age and gender did not differ between TRA and TFA groups, respectively. In the lowest tertile CardShock subgroup, TFA patients had lower BMI compared to TRA. TFA patients were more likely to be treated with higher number of vasopressors in the intermediate tertile, and they had lower systolic blood pressure at presentation in the highest tertile. TRA patients were more likely to present from an outside institution, had lower utilization of vasopressors (72.0 vs. 90.1%), and had a lower rate of escalation of MCS device therapy from the intra-aortic balloon pump (15.9% vs. 26.7%). Hemometabolic threshold markers at 24 hr were similar between TRA and TFA, but lactate levels were lower in TRA patients in the intermediate and high-risk tertiles. Other baseline clinical and hemodynamic characteristics did not differ by choice of coronary access site within each tertile.
TABLE 1.
Baseline characteristics of the study population stratified by tertiles of CardShock risk score and access site
| CardShock tertile 1 (n = 55) | CardShock tertile 2 (n = 54) | CardShock tertile 3 (n = 44) | ||||
|---|---|---|---|---|---|---|
| Parameter | Radial (n = 41) | Femoral (n = 14) | Radial (n = 31) | Femoral (n = 23) | Radial (n = 10) | Femoral (n = 34) |
| Demographics | ||||||
| Age, years | 66 (60, 70) | 64.5 (52, 68)NS | 63 (57, 72) | 66 (60,72)NS | 63.5 (52, 74) | 69 (63, 75)NS |
| Male | 34 (82.9) | 10 (71.4)NS | 21 (67.7) | 17 (73.9)NS | 7 (70.0) | 21 (61.8)NS |
| BMI median (Q1, Q3) | 28.4 (24.6, 32.3) | 23.4 (21.8, 27)* | 28.9 (25.7, 33) | 28.2 (23, 30.2)NS | 32.9 (31.3, 35.1) | 28.1 (24.5, 33.1)NS |
| Cerebrovascular disease | 0 (0.0) | 1 (7.1)NS | 1 (3.2) | 5 (21.7)* | 4 (40.0) | 13 (38.2)NS |
| Clinical characteristics | ||||||
| Diabetes mellitus | 16 (39.0) | 4 (28.6)NS | 13 (41.9) | 10 (43.4)NS | 6 (60.0) | 23 (67.7)NS |
| Index GFR < 60 ml/min | 17 (41.5) | 5 (35.7)NS | 17 (54.8) | 17 (73.9)NS | 6 (60.0) | 26 (76.5)NS |
| Prior MI/PCI/valve surgery | 6 (14.6) | 3 (21.4)NS | 5 (16.1) | 7 (30.4)NS | 2 (20.0) | 6 (17.6)NS |
| EF ≤ 30% | 20 (48.8) | 9 (64.3)NS | 18(58.1) | 17 (73.9)NS | 5 (50.0) | 24 (70.6)NS |
| Mechanical ventilation | 14 (34.1) | 9 (64.3)* | 18 (58.0) | 20 (87.0)* | 10 (100) | 34 (100)NS |
| Number of vasopressors | 1 (0, 1) | 1 (1, 2)NS | 1 (1, 2) | 2 (1, 3)** | 3 (2, 3) | 3 (3, 3)NS |
| Systolic BP | 90 (87, 95) | 93.5 (82, 100)NS | 90 (80, 100) | 75 (70, 95)** | 75 (70, 82) | 70 (60, 75)* |
| Cardiac arrest | 6 (14.6) | 5 (35.7)NS | 10 (32.2) | 10 (43.5)NS | 3 (30.0) | 17 (50.0)NS |
| Smoking history | 26 (63.4) | 7 (50.0)NS | 15 (48.4) | 12 (52.2)NS | 6 (60.0) | 6 (17.6)NS |
| Severity of shock and hemodynamics | ||||||
| CardShock score | 3 (2,3) | 3 (2, 3)NS | 5 (4, 5) | 5 (4,6)NS | 6 (6, 7) | 7 (6, 7)NS |
| RA | 12 (10, 19) | 12 (10, 16)NS | 13 (11, 15) | 15 (10, 20)NS | 16 (14, 17) | 15 (11,18)NS |
| PCWP | 20 (16, 26.5) | 20 (19, 30)NS | 23 (18, 31)NS | 24 (16, 30)NS | 22 (21, 30) | 24 (15, 27)NS |
| RA/wedge ratio | 0.6 (0.6, 0.7) | 0.6 (0.4, 0.7)NS | 0.6 (0.4, 0.7) | 0.6 (0.5, 0.8)NS | 0.6 (0.55, 0.6) | 0.6 (0.5, 0.8)NS |
| Baseline lactate | 2 (1.3, 2.8) | 2.1 (1.5, 3.5)NS | 2.75 (1.9, 6.4) | 3.2 (2.1, 6.4)NS | 3.8 (1.7, 5.1) | 3.9 (2.2, 7.4)NS |
| Baseline CPO | 0.8 (0.6, 0.9) | 0.7 (0.4, 0.8)NS | 0.7 (0.6, 0.9) | 0.9 (0.6, 1.0)NS | 0.8 (0.7, 0.8) | 0.6 (0.5, 0.7)NS |
| Baseline PAPi | 1.4 (0.9, 2.4) | 1.1 (0.7, 1.7)NS | 1.4 (1.0, 2.0) | 1.2 (0.8, 1.8)NS | 1.2 (0.9, 1.6) | 1.8 (0.8, 2.1)NS |
| SCAI shock | ||||||
| C | 41 (100) | 14 (100)NS | 27 (87.1) | 13 (56.5)** | 0 (0.0) | 0 (0.0)NS |
| D | 0 (0.0) | 0 (0.0) | 4 (12.9) | 10 (43.5) | 5 (50.0) | 21 (61.8) |
| E | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (50.0) | 13 (38.2) |
| Clinical presentation | ||||||
| NSTEMI | 20 (48.8) | 5 (35.7)NS | 13 (41.9) | 12 (52.2)NS | 5 (50.0) | 16 (47.1)NS |
| STEMI | 20 (48.8) | 9 (64.3) | 17 (54.8) | 11 (47.8) | 5 (50.0) | 18 (52.9) |
| Unstable angina | 1 (2.4) | 0 (0.0)NS | 1 (3.2) | 0 (0.0)NS | 0 (0.0) | 0 (0.0)NS |
Note: Values presented are median (Q1, Q3) or frequency (percent), where appropriate.
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CPO, cardiac power output; EF, ejection fraction; NS, nonsignificant; NSTEMI, non-ST segment elevation myocardial infarction; PAPi, pulmonary arterial pulsatility index; PCI, percutaneous coronary intervention; PCWP, pulmonary capillary wedge pressure; RA, right atrium; SCAI, Society for Cardiovascular Angiography and Interventions; STEMI, ST-elevation myocardial infarction.
p < .05.
p < .01.
3.2 |. Coronary angiography and PCI
For all patients, median time to coronary vascular access was 14.0 min, which along with times to first balloon inflations, was consistent across tertiles, except the intermediate tertile, in which median time to coronary access was greater in the TFA cohort (15 vs. 12 min; p < .01). Drug eluting stents comprised the majority of intervention (69.3%) followed by medical therapy (12.4%) and CABG (11.8%). For both TRA and TFA groups, likelihood of CABG decreased significantly with increasing CS severity.
3.3 |. Complication rates
Fifty patients experienced a MACCE, and the majority of events (78.0%) occurred in the TFA patients in CardShock tertiles 2 and 3 (Table 2). Overall, 57 (37.3%) patients died within 30 days. All deaths occurred within tertiles 2 and 3 and of those, 44 (77.2%) patients were in the TFA group. Among all patients, 41 (26.7%) total bleeding events were observed, 32% of which were at the coronary access site, and 92% were those undergoing TFA. Remaining events included secondary vascular access-site bleeding (BARC 3a, 3b, 5) from TFA for MCS (n = 17) and nonvascular access-site bleeding (BARC 3c) (n = 11; 5 gastrointestinal bleeds, 2 hemothoraces resulting from traumatic cardiopulmonary resuscitation, 2 intracerebral bleeds, 1 pericardial effusion, and 1 traumatic scalp laceration following cardiac arrest with collapse). A total of 15 vascular access-site complications were noted, 12 of which occurred during large-bore TFA for MCS insertion (6 femoral artery bleeds requiring surgical repair, 3 ischemic lower limbs from occlusive large-bore sheaths [treated with percutaneous distal perfusion bypass circuit], 2 retroperitoneal bleeds [1 treated with covered stenting of the ipsilateral external iliac artery] and 1 lower extremity compartment syndrome requiring surgical cutdown and fasciotomy). Three patients in the TRA cohort developed hematomas from radial artery injury, which were treated conservatively with compression bandage without compartment syndrome or need for further therapy. There were a total of 6 PCI-related complications (3 flow-limiting coronary edge dissections, 3 acute stent thromboses) with 3 events in the TRA cohort and 3 in the TFA arm.
TABLE 2.
Angiographic and interventional characteristics stratified by CardShock risk score tertile
| CardShock tertile 1 (n = 55) | CardShock tertile 2 (n = 54) | CardShock tertile 3 (n = 44) | ||||
|---|---|---|---|---|---|---|
| Parameter | Radial N = 41 | Femoral N = 14 | Radial N = 31 | Femoral N = 23 | Radial N = 10 | Femoral N = 34 |
| Time to access, min | 14 (12, 18) | 13.5 (11, 15)NS | 12 (10, 15) | 15 (13, 18)** | 13.5 (12, 16) | 15 (14, 18)NS |
| Time to balloon, min | 24 (21, 27) | 23.5 (21, 27.5)NS | 22 (21, 25) | 25.5 (20.5, 28.5)NS | 24 (22, 27) | 24.5 (22, 27)NS |
| Contrast volume, cc | 115 (100, 140) | 130 (90, 175)NS | 130 (90, 180) | 160 (120, 189)NS | 160 (135, 260) | 150 (110, 180)NS |
| Fluoroscopy time, min | 12 (9, 15) | 16.7 (10, 23)* | 14 (9, 20) | 15 (8.5, 19)NS | 15.5 (9.5, 32) | 16 (10, 23)NS |
| Intervention | ||||||
| DES | 25 (61.0) | 9 (64.3) | 22 (71.0) | 20 (37.0) | 8 (80.0) | 22 (64.7) |
| BMS | 1 (2.4) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (8.8) |
| PTCA | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (2.9) |
| CABG | 9 (22.0) | 2 (14.3) | 6 (19.4) | 1 (4.3) | 0 (0.0) | 0 (0.0) |
| Medical management | 5 (12.2) | 1 (7.1) | 2 (6.5) | 1 (4.3) | 1 (10.0) | 8(23.5) |
| Anticoagulant | ||||||
| Bivalirudin | 2 (4.9) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 0 (0.0) | 0 (0.0) |
| UF heparin | 39 (95.1) | 14 (100)NS | 31 (100) | 21 (91.3)NS | 10 (100) | 34 (100)NS |
| Antiplatelet | ||||||
| Clopidogrel | 16 (39.0) | 5 (35.7) | 10 (32.3) | 13 (56.5) | 7 (70.0) | 14 (41.2) |
| Prasugrel | 4 (9.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ticagrelor | 12 (29.3) | 7 (50.0) | 12 (38.7) | 8 (34.8) | 3 (30.0) | 13 (38.2) |
| NA | 9 (21.9) | 2 (14.3)NS | 9 (29.0) | 2 (8.7)NS | 0 (0.0) | 7 (20.6)NS |
| IIbIIIa | 10 (24.4) | 5 (28.6)NS | 11 (35.4) | 8 (34.8)NS | 5 (50.0) | 11 (32.4)NS |
| Conversion radial to femoral | 1 (2.4) | 0 (0.0)NS | 0 (0.0) | 0 (0.0)NS | 0 (0.0) | 0 (0.0)NS |
| PCI complication | 1 (2.4) | 1 (3.2)NS | 1 (3.2) | 2 (8.7)NS | 1 (10.0) | 0 (0.0)NS |
| Multi-vessel PCI | 1 (2.4) | 1 (7.1)NS | 1 (3.2) | 2 (8.7)NS | 4 (40.0) | 3 (8.8)NS |
| Ultrasound for CA site | 1 (2.4) | 10 (71.4) | 5 (16.1) | 15 (65.2)** | 2 (20.0) | 25 (73.5)* |
| Ultrasound for MCS | 27 (65.9) | 9 (64.3)NS | 22 (71.0) | 12 (52.2)NS | 3 (30.0) | 15 (44.1)NS |
| Devices | ||||||
| IABP | 27 (65.9) | 11 (78.6)NS | 15 (48.4) | 10 (43.5)NS | 0 (0.0) | 13 (38.2)* |
| Escalation from IABP | 7 (25.9) | 3 (27.2)NS | 6 (40.0) | 5 (50.0)NS | 0 (0.0) | 11 (84.6)* |
| Impella only | 13 (31.7) | 4 (28.6)NS | 9 (29.0) | 10 (43.5)NS | 5 (50.0) | 18 (52.9)NS |
| Impella CP | 13 (100) | 4 (100) | 8 (88.9) | 10 (100) | 5 (100) | 18 (100) |
| Impella 2.5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Impella 5.0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Impella RP | 0 (0.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| VA-ECMO only | 0 (0.0) | 0 (0.0)NS | 2 (6.5) | 1 (4.4)NS | 0 (0.0) | 4 (11.8)NS |
| Impella + VA-ECMO | 1 (2.4) | 0 (0.0)NS | 4 (12.9) | 3 (13.0)NS | 0 (0.0) | 8 (23.5)* |
| Impella CP + VA-ECMO | 1 (100) | 0 (0.0) | 4 (100) | 3 (100) | 0 (0.0) | 7 (87.5) |
| Impella 5.0 + VA-ECMO | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5)NS |
| RA @ 24 hr | 10.7 (8, 10.7) | 13.3 (10, 13.3)* | 10.7 (9, 12) | 13 (10, 13.3)NS | 12.5 (10.7,13) | 13.3 (13.3,19)NS |
| PCWP @ 24 hr | 17.6 (16, 18) | 19 (18, 19)* | 17.6 (17.6, 19) | 19 (18, 19)NS | 17.6 (17.6, 18) | 19 (19, 20)NS |
| RA/PCWP @ 24 hr | 0.6 (0.4, 0.7) | 0.2 (0.1, 0.6)NS | 0.6 (0.5, 0.7) | 0.7 (0.4, 0.7)NS | 0.8 (0.6, 1.1) | 0.8 (0.7, 1)NS |
| Lactate @ 24 hr | 2.6 (1.3, 2.6) | 3.4 (1.6, 4.4)NS | 2.7 (1.5, 2.6) | 4.4 (1.8, 4.4)** | 1.8 (1.4, 2.6) | 4.4 (1.9, 5.2)* |
| CPO @ 24 hr | 0.9 (0.73, 1.1) | 0.8 (0.8, 0.8)NS | 0.9 (0.8, 0.9) | 0.8 (0.8, 0.9)NS | 0.9 (0.9, 1) | 0.8 (0.6, 0.8)NS |
| Papi @ 24 hr | 2.2 (2.1, 2.8) | 2.3 (2.3, 2.3)NS | 2.3 (1.5, 2.8) | 2.3 (1.2, 2.3)NS | 1.6 (0.8, 2.2) | 2 (1, 2.3)NS |
| Outcomes | ||||||
| Mortality, 30 day | 0 (0.0) | 0 (0.0)NS | 3 (9.7) | 12 (52.2)* | 10 (100) | 32 (94.1)NS |
| MACCE | 1 (2.4) | 0 (0.0)NS | 5 (16.1) | 13 (56.5)** | 5 (50.0) | 26 (76.5)NS |
| Coronary access-site bleed | 0 (0.0) | 3 (21.4)** | 0 (0.0) | 4 (17.4)** | 1 (10.0) | 5 (14.7)NS |
| Secondary vascular access-site bleed | 3 (7.3) | 0 (0.0)NS | 3 (9.7) | 4 (17.4)NS | 4 (40.0) | 3 (8.8)NS |
| Non-vascular access-site bleed | 1 (2.4) | 2 (14.3)NS | 1 (3.2) | 2 (8.7)NS | 1 (10.0) | 4 (11.8)NS |
| Vascular complications | 0 (0.0) | 2 (14.3)NS | 1 (3.2) | 3 (13.0)NS | 2 (20.0) | 7 (20.6)NS |
Note: Values presented are median (Q1, Q3) or frequency (percent), where appropriate.
Abbreviations: AKI, acute kidney injury; BMS, bare metal stent; CA, coronary access; CABG, coronary artery bypass graft surgery; DES, drug eluting stent; IABP, intra-aortic balloon pump; LVAD; left ventricular assist device; MACCE, major adverse cardiac and cerebrovascular events; NS, not significant; PCI, percutaneous coronary intervention; PCWP, pulmonary capillary wedge pressure; PTCA, percutaneous transluminal angioplasty; RA, right atrium; RHC, right heart catheterization, VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
p < .05.
p < .01.
3.4 |. SCAI shock staging system
We similarly describe the study patient’s baseline and interventional characteristics based on their SCAI staging at time of index CS diagnosis (Tables 3 and 4). Patients with SCAI CS stages D and E had higher rates of baseline diabetes and renal insufficiency, and they were more likely to undergo TFA. They also had higher baseline lactic acid levels and right atrial pressures. They were less likely to undergo CABG surgery than stage C CS. Patients with SCAI stages D and E CS were also more likely to be supported with veno-arterial extracorporeal membrane oxygenation and they had higher rates of 30-day mortality and major adverse cardiac and cerebrovascular events than stage C CS.
TABLE 3.
Baseline characteristics of the study population stratified by index SCAI shock classification
| Parameter | SCAI C N = 95 | SCAI D N = 40 | SCAI E N = 18 |
|---|---|---|---|
| Access site | |||
| Radial | 68 (71.6) | 9 (22.5) | 5 (27.8) |
| Femoral | 27 (28.4) | 31 (77.5) | 13 (72.2)† |
| Demographics | |||
| Age, years | 66 (58, 70) | 69 (61.5, 75.5) | 65 (58, 71) NS |
| Male | 72 (75.8) | 29 (72.5) | 9 (50.0) NS |
| BMI median (Q1, Q3) | 27.4 (23.8, 32.2) | 29.32 (24, 31.75) | 30.25 (26, 35.7) * |
| Cerebrovascular disease | 2 (2.1) | 15 (37.5) | 7 (38.9)† |
| Clinical characteristics | |||
| Diabetes | 33(34.7) | 26(65.0) | 13(72.2)† |
| Index GFR < 60 ml/min | 45 (47.4) | 27 (67.5) | 16 (88.9) ** |
| Prior MI/PCI/CABG/valve surgery | 20(21.1) | 4(10.0) | 5(27.8) NS |
| EF ≤ 30% | 53 (55.8) | 30 (75.0) | 10 (55.6) NS |
| Mechanical ventilation | 48 (50.5) | 39 (97.5) | 18 (100)† |
| Number of vasopressors | 1 (0, 2) | 2 (2, 3) | 3 (3, 3) NS |
| Systolic BP | 90 (85,100) | 73 (67, 78.5) | 70 (65, 75) NS |
| Cardiac arrest | 27 (28.4) | 12 (30.0) | 12 (66.7) ** |
| Smoking history | 54 (56.8) | 13 (32.5) | 5 (27.8) ** |
| RA | 12 (10, 17) | 14 (10, 18) | 16 (15, 18) * |
| PCWP | 20 (15, 27) | 24 (16, 28) | 21 (20, 24) NS |
| RA/wedge ratio | 0.6 (0.5, 0.7) | 0.6 (0.5, 0.8) | 0.8 (0.6, 1.0) NS |
| Baseline lactate | 2.5 (1.5, 4.2) | 3.4 (2.2, 4.7) | 7.1 (2.4, 11.9) ** |
| Baseline CPO | 0.7 (0.6, 0.9) | 0.7 (0.5, 0.8) | 0.6 (0.5, 0.8) NS |
| Baseline PAPi | 1.4 (0.9, 2) | 1.2 (0.9, 2.0) | 0.6 (0.4, 1) NS |
| Clinical presentation | |||
| NSTEMI | 42 (44.2) | 20 (50.0) | 9 (50.0) |
| STEMI | 51 (53.7) | 20 (50.0) | 9 (50.0) NS |
| Unstable angina | 2 (2.1) | 0 (0.0) | 0 (0.0) NS |
Note: Values presented are median (Q1, Q3) or frequency (percent), where appropriate.
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CPO, cardiac power output; EF, ejection fraction; NS, nonsignificant; NSTEMI, non-ST segment elevation myocardial infarction; PAPi, pulmonary arterial pulsatility index; PCI, percutaneous coronary intervention; PCWP, pulmonary capillary wedge pressure; RA, right atrium; SCAI, Society for Cardiovascular Angiography and Interventions; STEMI, ST-elevation myocardial infarction.
p < .05.
p < .01.
p < .001.
TABLE 4.
Angiographic and interventional characteristics stratified by index SCAI shock classification
| Parameter | SCAI C N = 95 | SCAI D N = 40 | SCAI E N = 18 |
|---|---|---|---|
| Time to access, min | 14 (11.5, 16.5) | 15 (12, 18) | 15 (14, 16) NS |
| Time to balloon, min | 23 (21, 26) | 24.5 (21, 27) | 24.5 (24, 29) NS |
| Contrast volume, cc | 120 (100, 160) | 160 (127.5, 190) | 130 (110, 160)† |
| Fluoroscopy time, min | 14 (9.8, 18) | 15.5 (9, 24.9) | 15.6 (10, 18.5)† |
| Intervention | |||
| DES | 65 (68.4) | 29 (72.5) | 12 (66.7) |
| BMS | 2 (2.1) | 3 (7.5) | 0 (0.0) |
| PTCA | 1 (1.1) | 0 (0.0) | 2 (11.1) |
| CABG | 17 (17.9) | 1 (2.5) | 0 (0.0) |
| Medical management | 7 (7.4) | 7(12.5) | 4 (22.2) ** |
| Anticoagulant | |||
| Bivalirudin | 3 (3.2) | 1 (2.5) | 0 (0.0) |
| UF heparin | 92 (96.8) | 39 (97.5) | 18 (100) NS |
| Antiplatelet | |||
| Clopidogrel | 37 (39.0) | 18 (45.0) | 10 (55.6) |
| Prasugrel | 4 (4.2) | 0 (0.0) | 0 (0.0) |
| Ticagrelor | 34 (35.8) | 16 (40.0) | 5 (27.8) |
| NA | 20 (21.1) | 6 (15.0) | 3 (16.7) NS |
| IIbIIIa | 28 (29.5) | 15 (37.5) | 7 (38.9) NS |
| PCI complication | 2 (2.1) | 3 (7.5) | 1 (5.6) NS |
| Multi-vessel PCI | 3 (3.2) | 6 (15.0) | 3 (16.8) * |
| Ultrasound for CA site | 30 (31.6) | 20 (50.0) | 8 (44.4) NS |
| Ultrasound for MCS | 62 (65.3) | 18 (45.0) | 8 (44.4) * |
| Devices | |||
| IABP | 59 (62.1) | 12 (30.0) | 5 (27.8)† |
| Escalation from IABP | 22 (37.3) | 7 (58.3) | 3 (60.0) NS |
| Impella only | 32 (33.7) | 18 (45.0) | 9 (50.0) NS |
| Impella CP | 32 (100) | 17 (94.4) | 9 (100) |
| Impella 2.5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Impella 5.0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Impella RP | 0 (0.0) | 1 (5.6) | 0 (0.0) NS |
| VA-ECMO only | 2 (2.1) | 1 (2.5) | 4 (22.2)† |
| Impella + VA-ECMO | 7 (7.4) | 6 (15.0) | 3 (16.7) NS |
| Impella CP + VA-ECMO | 7 (100) | 6 (100) | 2 (66.6) |
| Impella 5.0 + VA-ECMO | 0 (0.0) | 0 (0.0) | 1 (33.3) |
| RA @ 24 hr | 10.7 (8, 13) | 13.3 (10.9, 17.5) | 13.3 (13.3, 19) * |
| PCWP @ 24 hr | 17.6 (16, 19) | 18.9 (17, 22.5) | 18.9 (18.9, 21) NS |
| RA/PCWP @ 24 hr | 0.6 (0.4, 0.7) | 0.8 (0.6, 1) | 0.9 (0.7, 0.9) NS |
| Lactate @ 24 hr | 2.6 (1.3, 2.6) | 2.8 (1.7, 4.4) | 4.4 (2.6, 12)† |
| CPO @ 24 hr | 0.9 (0.8, 1.0) | 0.8 (0.7, 0.9) | 0.8 (0.8, 0.8) NS |
| Papi @ 24 hr | 2.2 (1.7, 2.8) | 1.7 (1.0, 2.3) | 2.2 (1.1, 2.25) NS |
| Outcomes | |||
| Mortality, 30 day | 4 (4.2) | 36 (90.0) | 17 (94.4)† |
| MACCE | 1 (1.1) | 31 (77.5) | 18 (100)† |
| Coronary access-site bleed | 5 (5.3) | 7(17.5) | 1 (5.6)† |
| Secondary vascular access-site bleed | 8 (8.4) | 5 (12.5) | 4 (22.2) NS |
| Non-vascular access-site bleed | 3 (3.2) | 3 (7.5) | 5 (27.8)† |
| Vascular complications | 2 (2.1) | 4 (10.0) | 9 (50.0)† |
Note: Values presented are median (Q1, Q3) or frequency (percent), where appropriate.
Abbreviations: AKI, acute kidney injury; BMS, bare metal stent; CA, coronary access; CABG, coronary artery bypass graft surgery; DES, drug eluting stent; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; MACCE, major adverse cardiac and cerebrovascular events; NS, not significant; PCI, percutaneous coronary intervention; PCWP, pulmonary capillary wedge pressure; PTCA, percutaneous transluminal angioplasty; RA, right atrium; RHC, right heart catheterization; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
p < .05.
p < .01.
p < .001.
3.5 |. Hierarchal logistic regression
Lastly, we performed a series (n = 3) of hierarchical logistic regression models to ascertain the effect of CS severity and TRA or TFA on a successful PCI after controlling for baseline characteristics (See Methods Section) (Table 5). In step 1 (model 1), younger age was a significant predictor of successful TIMI III flow PCI and survival at 30 days (OR: 0.78; 95% CI: 0.63–0.96). Following the statistically significant addition of CardShock risk score tertiles, successful PCI was more likely in patients in the first and second tertiles as compared to the sickest patients in tertile 3 (CardShock tertile 1 vs. 3: OR: 18.61; 95% CI: 2.86–121.25 and (Cardshock tertile 2 vs. 3: OR: 16.21; 95% CI: 2.91–90.37) (Figure 2). However, after adding the choice of coronary access site in level-3 of the hierarchical model, no statistically significant difference was observed between of use of TRA versus TFA (OR: 1.36; 95% CI: 0.54–3.40).
TABLE 5.
Hierarchical multivariate odds ratios and confidence intervals for successful PCI
| Model | Parameter | OR | 95% CI | p | R2 | ΔR2 | AIC | ΔAIC | C-Statistic |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Age, per 5 years | 0.77 | (0.63–0.96) | .0168 | - | - | - | - | - |
| Female | 0.43 | (0.18–1.06) | .0683 | - | - | - | - | - | |
| BMI, kg/m2 | 0.94 | (0.88–1.01) | .0911 | - | - | - | - | - | |
| Systolic BP at baseline, mmHg | 1.01 | (0.97–1.05) | .7349 | - | - | - | - | - | |
| Number of pressors | 0.64 | (0.37–1.05) | .0808 | - | - | - | - | - | |
| Cardiac arrest | 0.73 | (0.29–1.87) | .5190 | - | - | - | - | - | |
| Intubated | 0.45 | (0.17–1.18) | .1035 | - | - | - | - | - | |
| Lactate at baseline | 0.99 | (0.87–1.12) | .8873 | - | - | - | - | - | |
| Prior CABG | 0.56 | (0.13–2.39) | .4098 | - | - | - | - | - | |
| MCS | 0.88 | (0.32–2.39) | .8007 | 0.243 | - | 185.907 | - | 0.795 | |
| 2 | CardShock risk tertile | ||||||||
| 1 | 18.77 | (2.90–121.65) | .0136 | - | - | - | |||
| 2 | 16.11 | (2.90–90.38) | .0116 | - | - | - | |||
| 3 | Ref | Ref | - | 0.312 | 0.069 | 175.122 | −10.785 | 0.820 | |
| 3 | Radial versus femoral | ||||||||
| Radial | 1.36 | (0.54–3.41) | .4345 | - | - | - |
Abbreviations: AIC, akaike information criterion; BMI, body mass index; CABG, coronary artery bypass graft; MCS, mechanical circulatory support; TIMI, thrombolysis in myocardial infarction.
FIGURE 2.
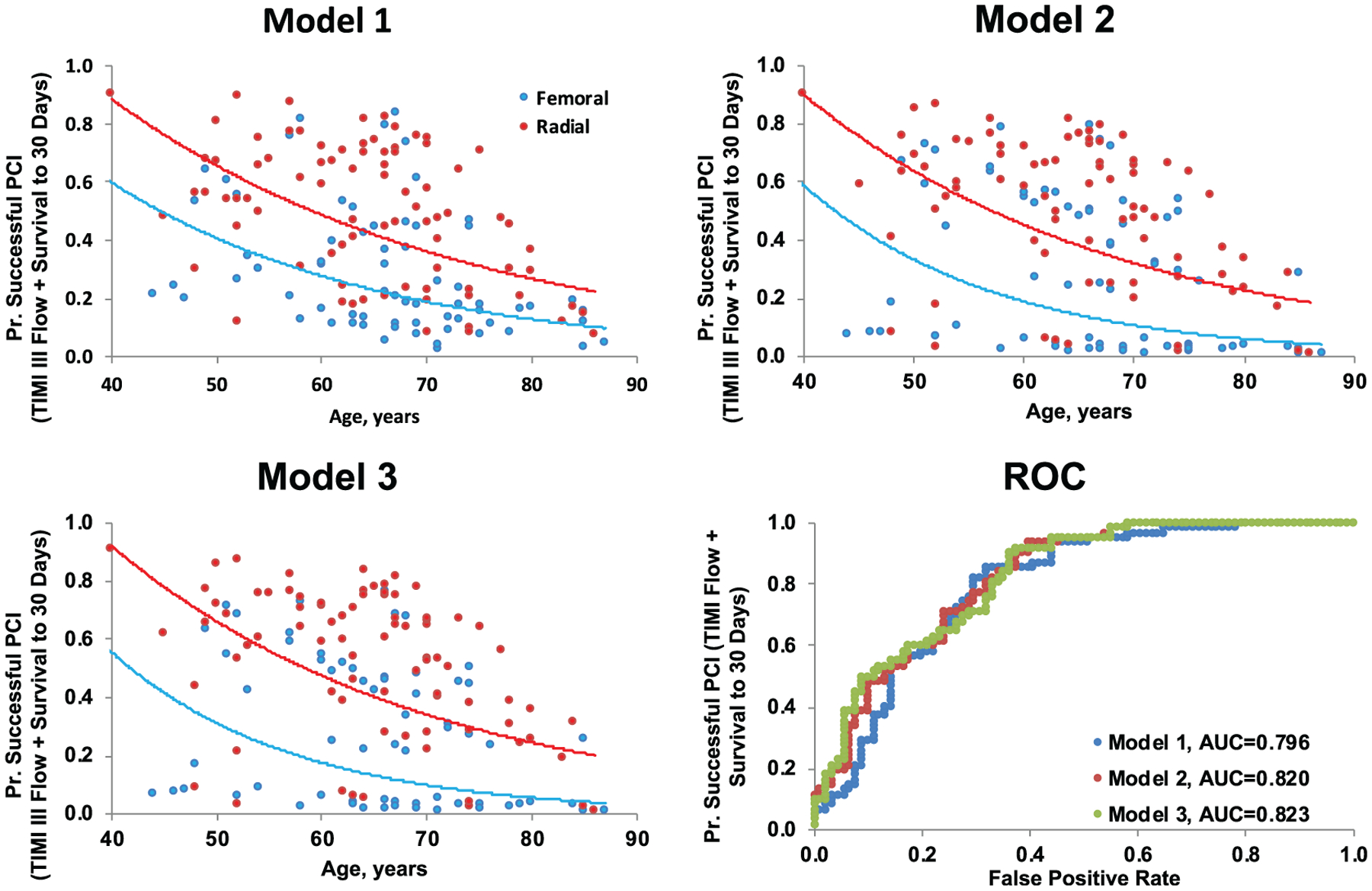
Multivariate predictors of successful PCI. Results of hierarchical multivariate regression model modeling the probability of successful PCI (Thrombolysis in Myocardial Infarction [TIMI] III flow and 30-day survival). Model 1 adjusted for age, sex, BMI, systolic BP at baseline, number of pressors, cardiac arrest, intubated, lactate at baseline, prior CABG, and mechanical circulatory support. Model 2 adjusted for model 1 parameters + CardShock tertile (ref = tertile 3). Model 3 adjusted for model 2 + radial/femoral access (ref = femoral)
4 |. DISCUSSION
The main findings from this observational study are: (a) TRA for coronary angiography and PCI is feasible in patients across the entire spectrum of AMI-CS, including those treated with axial-and centrifugal-flow MCS devices; (b) There is no difference between TRA and TFA in achieving successful percutaneous coronary revascularization and 30-day survival in AMI-CS; and (c) TRA is associated with reduced coronary access-site bleeding in patients with AMI-CS.
Prior evidence from observational studies suggesting clinical merit to TRA in AMI-CS has been limited by lack of external validation and CS stratification by disease severity.9,10 In the Culprit Lesion Only PCI versus Multi-vessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial, for example, 89% of the AMI-CS patients were on vasopressors, 81% were mechanically ventilated, and 53% had been resuscitated from cardiac arrest with median time of 3 days to hemodynamic stabilization.23 While the authors did not adjudicate the relationship between access-site utilization and clinical end points, TRA was utilized in only 19% of the patients, suggesting that acuity of illness and consideration of large-bore access for possible MCS may dissuade operators from accessing the radial artery. Our published series is the first to describe arterial access-site selection in patients with AMI-CS stratified and treated based on disease severity using standardized protocols and validated risk scores, which consider clinical demographics as well as serial laboratory and invasive hemodynamic markers.1,11,19,24
Contemporary analyses of single and multicenter studies confirm that the incidence of AMI-CS is on the rise, and the patients are increasingly complex with greater burden of comorbidities and hemometabolic derangements.25,26 In the Danish RETROSHOCK registry, for example, patients admitted with diagnosis of AMI-CS between 2013 and 2017 had greater need for vasoactive therapies, mechanical ventilation, and hemodialysis compared to counterparts from 2010 to 2012.26 In addition, those treated with MCS were more likely to be supported with axial- and centrifugal-flow devices compared to the conventional IABP, and they had higher rates of major bleeding and transfusion requirements (58.6 vs. 31.5%; p < .001); these are independent predictors of increased mortality following PCI.26,27 Our study demonstrated similar findings, as patients with SCAI stages D and E CS were more likely to present with cardiac arrest, and they also had higher lactic acid levels and right atrial pressures 24 hr following initiation of therapies. They were also more likely to undergo escalation of MCS from the IABP and they had higher rates of major vascular complications and 30-day mortality. These findings highlight the enhanced risk for bleeding and associated complications with large-bore access, which can be seen with escalating severities of CS. We did, however, demonstrate that the use of US reduces the risk of bleeding and vascular complications in this critically ill patient population. While this study was not powered to detect a clinical benefit with US guidance for coronary access-site bleeding among TRA patients, we noted reductions in coronary access-site bleeding (8.5 vs. 33.0%, p = .01), secondary noncoronary access-site bleeding (6.8 vs. 31.4%, p < .001), and vascular complications (8.1 vs. 41.7%, p < .001) among patients undergoing US-guided TFA when compared to non-US-guided access, without significant delays in median access times (15.0 min [14, 18] versus 15.0 min [12, 18]). Consistent with prior studies and guideline recommendations highlighting the clinical merits of US guidance in contemporary practice, our findings highlight the need for standardizing practices for vascular access in cardiac catheterization laboratories, regardless of acuity of illness, variations in operator experience, and perceptions regarding the potential utility of adjuvant imaging technology.17,18,28
Our study also demonstrated that TRA is technically feasible in AMI-CS, with only 1.2% cross over rate to TFA, and it was not associated with appreciable increases in time to arterial access, balloon inflation, fluoroscopy times, contrast utilization, or PCI-related complications, compared to TFA. While we did note a reduction in TRA utilization in patients with increasing severity of illness based on CardShock and SCAI CS staging, likely driven by selection bias due to systemic hypoperfusion and potential need for MCS, we did demonstrate that TRA can be associated with significant reductions in major coronary access-site bleeding in low-to-intermediate risk CardShock AMI-CS patients. In addition, we also demonstrated using a hierarchical multivariable regression model incorporating 10 clinically relevant factors interventionalists encounter in the contemporary care of CS patients that there exists clinical equipoise between the two access sites in facilitating a successful PCI (Figure 3). These are noteworthy findings and provide further insight into the clinical utility of TRA in modulating the risk for bleeding and adverse clinical outcomes in AMI-CS patients requiring the full spectrum of acute cardiovascular care.29
FIGURE 3.
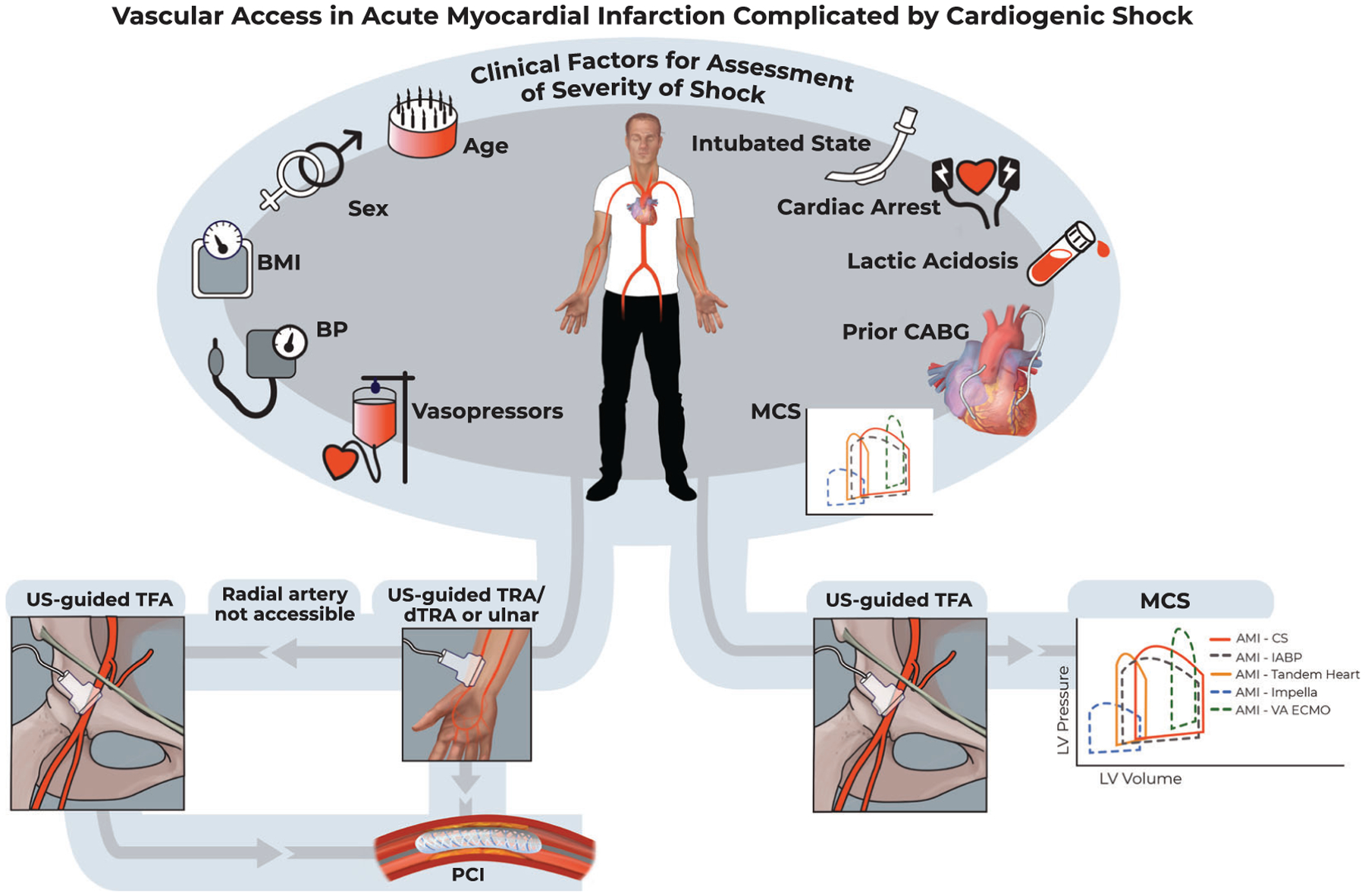
Vascular access in acute myocardial infarction complicated by cardiogenic shock. BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft surgery; dTRA, distal transradial access; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; TFA, transfemoral access; TRA, transradial access; US, ultrasound; VA-ECMO, veno-arterial extracorporeal membrane oxygenation
4.1 |. Study limitations
This was a nonrandomized single-center prospective registry with a limited number of patients, which may affect multilevel logistic regression modeling and statistical power of the study.30 In addition, the observational nature of this study lends itself to the inherent selection biases, which can be seen with lethal syndromes such as CS. Nevertheless, this is the first published series to describe coronary access-site selection in patients with AMI-CS using objective and validated disease severity definitions. In addition, we believe that our registry population is representative of clinical practice at contemporary quaternary care medical centers, given the breadth of clinical acuity and need for full spectrum MCS.24 Our all-comer registry also included patients with the highest severity of CS, SCAI Stage E, all of whom were intubated, 89% had chronic kidney disease, and they had median lactates of 4.4 mmol/L and evidence of biventricular congestion at 24 hr, with a >90% 30-day mortality rate. Despite the feasibility of TRA in these patients, they have sometimes been excluded from other AMI-CS registries, and further research is needed to better understand the potential benefits of coronary revascularization in end-stage CS.
5 |. CONCLUSION
This observational study provides evidence that TRA is a viable arterial access site across the entire spectrum of AMI-CS. It is associated with significant reductions in coronary access-site bleeding compared to TFA in low and intermediate risk tertiles. In addition, US-guided TFA can help to mitigate the risk of coronary and secondary access-site bleeding as well as vascular complications. Concerted efforts should be made to incorporate vascular access best practices into existing CS treatment protocols in dedicated shock care centers.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Dudley Family for their continued contributions and support of the INOVA Dudley Family Center for Cardiovascular Innovation. The authors would also like to acknowledge Ms. Devon Stuart for her artistic contribution to Figure 3.
CONFLICT OF INTEREST
B. N. T. received consulting and speaker honoraria from Medtronic. A. A. D. received research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (OAIC) Funded by the National Institute on Aging (NIA) P30-AG021334. A. G. T. received consulting and speaker honoraria from Abiomed. W. B. B. received speaker honoraria from Boston Scientific, Abbott Medical, and Medtronic.
Abbreviations:
- AMI
acute myocardial infarction
- CABG
coronary artery bypass graft
- CS
cardiogenic shock
- MCS
mechanical circulatory support
- NSTEMI
non-ST elevation myocardial infarction
- PCI
percutaneous coronary intervention
- TFA
transfemoral access
- TRA
transradial access
- US
ultrasound
Footnotes
This study was presented at the Transcatheter Cardiovascular Therapeutics 31st Annual Scientific Session, San Francisco, CA.
REFERENCES
- 1.Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73(13): 1659–1669. [DOI] [PubMed] [Google Scholar]
- 2.Damluji AA, Myerburg RJ, Chongthammakun V, et al. Improvements in outcomes and disparities of ST-segment-elevation myocardial infarction care: the Miami-Dade County ST-segment-elevation myocardial infarction network project. Circ Cardiovasc Qual Outcomes. 2017;10(12). e004038. [DOI] [PubMed] [Google Scholar]
- 3.Damluji AA, Bandeen-Roche K, Berkower C, et al. Percutaneous coronary intervention in older patients with ST-segment elevation myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2019;73(15):1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4(6): 654–664. [DOI] [PubMed] [Google Scholar]
- 5.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella(R) use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2019;141:273–284. [DOI] [PubMed] [Google Scholar]
- 6.Mason PJ, Shah B, Tamis-Holland JE, et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. 2018;11(9): e000035. [DOI] [PubMed] [Google Scholar]
- 7.Kopin D, Seth M, Sukul D, et al. Primary and secondary vascular access site complications associated with percutaneous coronary intervention: insights from the BMC2 registry. JACC Cardiovasc Interv. 2019;12:2247–2256. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante G, Rao SV, Juni P, et al. Radial versus femoral access for coronary interventions across the entire Spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv. 2016;9(14):1419–1434. [DOI] [PubMed] [Google Scholar]
- 9.Pancholy SB, Shantha GPS, Romagnoli E, et al. Impact of access site choice on outcomes of patients with cardiogenic shock undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Am Heart J. 2015;170(2):353–361. [DOI] [PubMed] [Google Scholar]
- 10.Mamas MA, Anderson SG, Ratib K, et al. Arterial access site utilization in cardiogenic shock in the United Kingdom: is radial access feasible? Am Heart J. 2014;167(6):900–8.e1. [DOI] [PubMed] [Google Scholar]
- 11.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37. [DOI] [PubMed] [Google Scholar]
- 12.Abdelaal E, Brousseau-Provencher C, Montminy S, et al. Risk score, causes, and clinical impact of failure of transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. 2013;6(11): 1129–1137. [DOI] [PubMed] [Google Scholar]
- 13.Patel N, Sharma A, Dalia T, et al. Vascular complications associated with percutaneous left ventricular assist device placement: a 10-year US perspective. Catheter Cardiovasc Interv. 2019;95(2):309–316. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, et al. Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial I. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018; 72(18):2231–2264. [DOI] [PubMed] [Google Scholar]
- 15.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9): 625–634. [DOI] [PubMed] [Google Scholar]
- 16.Rao SV, Tremmel JA, Gilchrist IC, et al. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention’s transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228–236. [DOI] [PubMed] [Google Scholar]
- 17.Sandoval Y, Burke MN, Lobo AS, et al. Contemporary arterial access in the cardiac catheterization laboratory. JACC Cardiovasc Interv. 2017;10(22):2233–2241. [DOI] [PubMed] [Google Scholar]
- 18.Damluji AA, Nelson DW, Valgimigli M, et al. Transfemoral approach for coronary angiography and intervention: a collaboration of international cardiovascular societies. JACC Cardiovasc Interv. 2017;10(22): 2269–2279. [DOI] [PubMed] [Google Scholar]
- 19.Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015; 17(5):501–509. [DOI] [PubMed] [Google Scholar]
- 20.Schrage B, Dabboura S, Yan I, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020. 10.1002/ccd.28707. [DOI] [PubMed] [Google Scholar]
- 21.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation. 2011;123:2737–2747. [DOI] [PubMed] [Google Scholar]
- 22.Wong GY, Mason WM. The hierarchical logistic regression model for multilevel analysis. J Am Stat Assoc. 1985;80(391):513–524. [Google Scholar]
- 23.Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419–2432. [DOI] [PubMed] [Google Scholar]
- 24.Basir MB, Kapur NK, Patel K, et al. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93(7):1173–1183. [DOI] [PubMed] [Google Scholar]
- 25.Garcia S, Schmidt CW, Garberich R, et al. Temporal changes in patient characteristics and outcomes in ST-segment elevation myocardial infarction 2003–2018. Catheter Cardiovasc Interv. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Helgestad OKL, Josiassen J, Hassager C, et al. Contemporary trends in use of mechanical circulatory support in patients with acute MI and cardiogenic shock. Open Heart. 2020;7(1):e001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok CS, Sherwood MW, Watson SM, et al. Blood transfusion after percutaneous coronary intervention and risk of subsequent adverse outcomes: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2015;8(3):436–446. [DOI] [PubMed] [Google Scholar]
- 28.Shroff AR, Gulati R, Drachman DE, et al. SCAI expert consensus statement update on best practices for transradial angiography and intervention. Catheter Cardiovasc Interv. 2020;95(2):245–252. [DOI] [PubMed] [Google Scholar]
- 29.Romagnoli E, De Vita M, Burzotta F, et al. Radial versus femoral approach comparison in percutaneous coronary intervention with intraaortic balloon pump support: the RADIAL PUMP UP registry. Am Heart J. 2013;166(6):1019–1026. [DOI] [PubMed] [Google Scholar]
- 30.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]


