Abstract
Objective
Obesity, in particular visceral obesity, and insulin resistance emerged as major risk factors for severe coronavirus disease 2019 (COVID-19), which is strongly associated with hemostatic alterations. Because obesity and insulin resistance predispose to thrombotic diseases, we investigated the relationship between hemostatic alterations and body fat distribution in participants at risk for type 2 diabetes.
Subjects
Body fat distribution (visceral and subcutaneous abdominal adipose tissue) and liver fat content of 150 participants – with impaired glucose tolerance and/or impaired fasting glucose – were determined using magnetic resonance imaging and spectroscopy. Participants underwent precise metabolic characterization and major hemostasis parameters were analyzed.
Results
Procoagulant factors (FII, FVII, FVIII, and FIX) and anticoagulant proteins (antithrombin, protein C, and protein S) were significantly associated with body fat distribution. In patients with fatty liver, fibrinogen (298 mg/dl vs. 264 mg/dl, p = 0.0182), FVII (99% vs. 90%, p = 0.0049), FVIII (114% vs. 90%, p = 0.0098), protein C (124% vs. 111%, p = 0.0006), and protein S (109% vs. 89%, p < 0.0001) were higher than in controls. In contrast, antithrombin (97% vs. 102%, p = 0.0025) was higher in control patients. In multivariate analyses controlling for insulin sensitivity, body fat compartments, and genotype variants (PNPLA3I148MM/MI/TM6SF2E167kK/kE), only protein C and protein S remained significantly increased in fatty liver.
Conclusions
Body fat distribution is significantly associated with alterations of procoagulant and anticoagulant parameters. Liver fat plays a key role in the regulation of protein C and protein S, suggesting a potential counteracting mechanism to the prothrombotic state in subjects with prediabetes and fatty liver.
Keywords: Body fat distribution, Abdominal obesity, Liver fat, Prediabetes, Coagulation
Highlights
-
•
Body fat distribution is associated with hemostatic alterations in subjects at risk for type 2 diabetes.
-
•
Procoagulant factors and anticoagulant proteins are elevated in subjects with fatty liver.
-
•
The link between liver fat and altered hemostasis is mainly driven by confounders like BMI and insulin sensitivity.
-
•
Only protein C and protein S were significantly and independently increased in subjects with fatty liver.
1. Introduction
During the coronavirus disease 2019 (COVID-19) pandemic, obesity and insulin resistance were identified as major risk factors for hospitalization and critical illness of patients with COVID-19 [1,2]. A hallmark of COVID-19 is the association with thrombotic diseases such as deep vein thrombosis and pulmonary embolism, which essentially contributes to the increased morbidity and mortality in severe COVID-19 [2,3]. Endothelial dysfunction and alterations in the plasmatic coagulation system are potential mechanisms explaining the prothrombotic tendency in COVID-19 [[4], [5], [6]]. Obesity and insulin resistance are also associated with a prothrombotic state predisposing to arterial and venous thrombosis [7,8]. Studies found increased activities of procoagulant parameters (e.g. FVII and FVIII) and endothelial dysfunction markers in obese patients that might explain the increased risk for thrombotic diseases [9,10].
The site of fat accumulation in the body is a key determinant for cardiometabolic risk [11,12]. Recent data suggest that visceral obesity is even a better predictor of the severity of COVID-19, compared to general obesity, but the underlying causes are unclear [13,14]. Considering liver fat, patients with nonalcoholic fatty liver disease (NAFLD) are also at increased risk for thrombotic diseases [15]. Recently, we revealed that a 1-year lifestyle intervention improves the prothrombotic tendency in subjects at increased risk for type 2 diabetes [16]. This is, at least partially, mediated by a reduction in liver fat content. However, the impact of fat accumulation in specific depots on the hemostasis system has only been addressed in a few studies, so far [8,17,18]. Subjects at increased risk for type 2 diabetes, of which fatty liver is common comorbidity [19], have not yet been studied in this context. Because most of the major hemostasis parameters are synthesized in the liver and ectopic fat has become a promising target for therapeutic interventions, it is of great interest to elucidate underlying associations between fatty liver and hemostatic alterations that may help to improve therapeutic strategies to reduce the cardiometabolic risk in these subjects [20].
Based on findings from COVID-19 patients, we now hypothesized that body fat distribution is a key determinant for alterations in the hemostasis system. Therefore, we investigated the relationship between the amount of visceral and subcutaneous abdominal fat, liver fat content, and established hemostasis parameters in subjects at risk for type 2 diabetes.
2. Subjects and methods
2.1. Study design and participants
In the present study, data from 150 subjects with impaired glucose tolerance and/or impaired fasting glucose were analyzed, who were randomly selected from the “Prediabetes Lifestyle Intervention Study” (PLIS) cohort (NCT01947595) [21]. Participants used for the present analysis were exclusively recruited from the University Hospital in Tübingen. Participants (N = 9) receiving vitamin K antagonists, direct oral anticoagulants, or estrogens were excluded owing to potential interferences with hemostasis assays. Further exclusion criteria included diabetes mellitus, previous thrombotic events, chronic kidney diseases, active malignant diseases, systemic infections, or elevated liver transaminases (>3x of the upper reference range). The study was performed at the Department of Internal Medicine and at the Institute for Clinical Chemistry and Pathobiochemistry of the University of Tübingen. The study was carried out in accordance with the declaration of Helsinki and was approved by the local Ethics Committee of the University of Tübingen (055/2012BO1). All participants provided written informed consent before study enrollment.
2.2. Genotpying
All genotyping was performed using a 700 k Infinium Global Screening Array from Illumina (SanDiego, CA, USA). Imputation was carried out on the Haplotype Reference Consortium reference panel using the Michigan Imputation Server. From the analyzed single nucleotide polymorphisms (SNPs), rs58542926 (TM6SF2) was genotyped and rs738409 (PNPLA3) has been imputed (R2 = 0.89).
2.3. Determination of body fat distribution and liver fat
MR examinations were performed in the early morning after an overnight fast on a 3 T whole-body imager (Magnetom Vida, Siemens Healthineers, Erlangen, Germany). MRI for the quantification of visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SCAT) was conducted using fuzzy means and an extended snake algorithm for segmentation of adipose and visceral adipose tissue according to previous publications [22,23]. Liver fat content was quantified in % by the ratio of lipids (methylene + methyl) and water + lipids by volume selective proton magnetic resonance spectroscopy (1H-MRS).
2.4. Laboratory measurements
Determination of laboratory parameters was performed using lithium heparin, sodium citrate, sodium fluoride, or clot activator-containing tubes (all from Sarstedt, Nümbrecht, Germany). All samples were collected after an overnight fast and immediately centrifuged after blood collection. Afterward, plasma supernatants were transferred into tubes which were stored at −80 °C until the determination of laboratory parameters.
Hemostasis measurements were performed using the following reagents from Siemens Healthineers: prothrombin time (PT): Dade Innovin; activated partial thromboplastin time (aPTT): Actin FS; D-Dimer: Innovance D-Dimer; fibrinogen: Dade Thrombin; FII and FVII: Dade Innovin and respective coagulation factor poor plasma; FVIII activity: FVIII chromogenic; FIX: Actin FS and respective coagulation factor poor plasma; antithrombin: Innovance antithrombin; protein C: Berichrom protein C (chromogenic); protein S (free antigen): Innovance free protein S antigen. The Atellica COAG 360 coagulation analyzer (Siemens Healthineers, Eschborn, Germany) was used as a platform for all coagulation measurements.
Clinical chemistry parameters (creatinine, glucose, alanine aminotransferase, aspartate aminotransferase, and C-reactive protein) were determined on an ADVIA XPT Clinical Chemistry system (Siemens Healthineers). Glycated hemoglobin (HbA1c) was determined using the Tosoh G8 HPLC analyzer (Tosoh Bioscience, Sursee, Switzerland).
2.5. Statistical analyses
Data are presented as median and interquartile range (1st – 3rd). Insulin sensitivity was calculated based on the results during an oral glucose tolerance test (OGTT) according to the formula by Matsuda and DeFronzo [24]. Correlation analyses given in Figure 1 were performed using the nonparametric Spearman's rank correlation coefficient (r). Linear regression analyses were performed to analyze the relationship between hemostasis parameters and body fat distribution, liver fat content, or metabolic variables. Results of clinical characteristics and laboratory parameters of participants with increased liver fat content (>5.56%) and control patients (liver fat content ≤5.56%) were compared using the Mann–Whitney U test [25]. Multiple linear regression analysis was performed to adjust for anthropometric and metabolic variables. SCAT and VAT were used as percent (%) of total adipose tissue in linear regression models comparing control patients and patients with increased liver fat content. A p-value <0.05 indicates statistical significance. To test for potential multicollinearity, the variation inflation factor (VIF) was used. Multicollinearity was assumed when VIF >5. All analyses were conducted using JMP software (V14.2.0, SAS Institute, Cary, United States). Figure 1 was created with GraphPad Prism Software (version 8). Figure 2 was created using Microsoft Office PowerPoint.
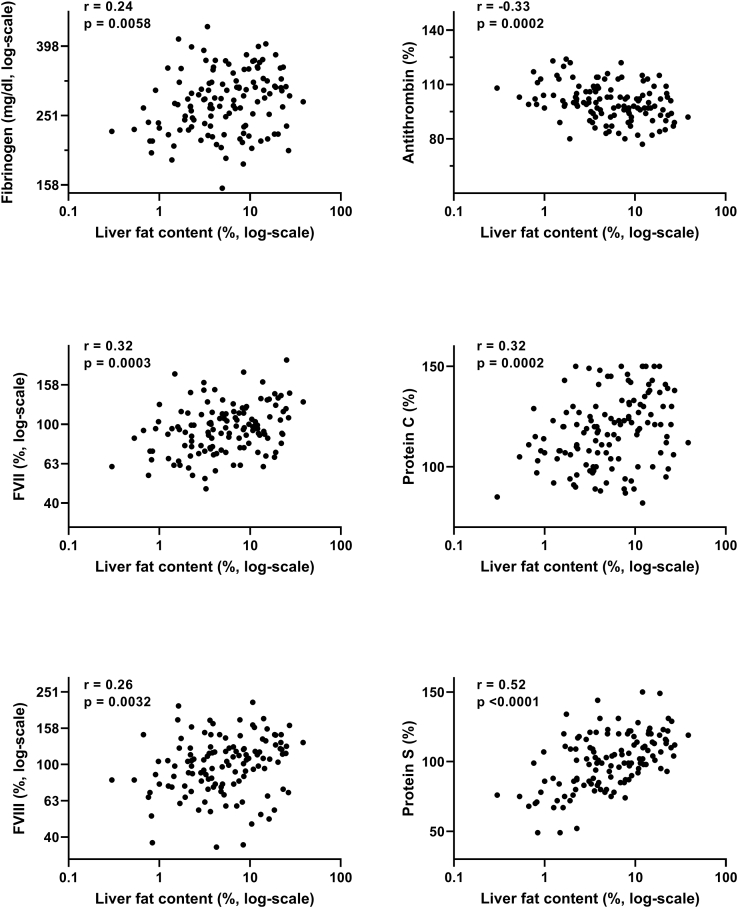
Figure 1.
Relationship between hemostasis parameters and liver fat content.
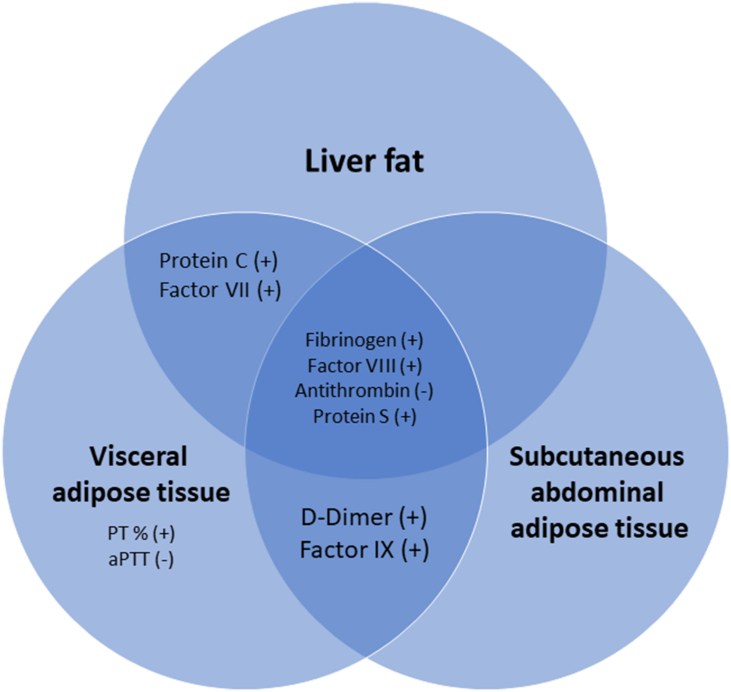
Figure 2.
Relationship between liver fat, visceral adipose tissue (VAT), and subcutaneous abdominal adipose tissue (SCAT) with hemostasis parameters in subjects at risk for type 2 diabetes. The indicated hemostasis parameters exhibit positive (+) or negative (−) associations with VAT, SCAT, and/or liver fat according to Table 2.
3. Results
3.1. Study population
A total of 141 patients with impaired fasting glucose and/or impaired glucose tolerance were included in the present study. Participants (85 women and 56 men) had a median age of 59 years (interquartile range (IQR): 52–65) and a median body mass index (BMI) of 30 kg/m2 (26–33). Determination of body fat distribution covered a wide range of total adipose tissue (TAT, IQR: 25.6–45.2 l), visceral adipose tissue (VAT, 3.2–6.5 l), subcutaneous abdominal adipose tissue (SCAT, 7.9–17.0 l), and liver fat content (2.4–11.6%). Detailed clinical and laboratory characteristics including body fat distribution and results of hemostasis measurements are summarized in Table 1.
Table 1.
Characteristics of the study participants.
| Anthropometric and laboratory characteristics | |
| Sex (w/m, n) | 85/56 |
| Age (years) | 59 (52–65) |
| Body weight (kg) | 84 (72–100) |
| Body-Mass-Index (kg/m2) | 30 (26–33) |
| Fasting glucose (mmol/l) | 5.8 (5.6–6.2) |
| OGTT-derived insulin sensitivity | 8.3 (5.8–12.5) |
| Glycated hemoglobin (HbA1c, mmol/mol) | 40 (38–43) |
| C-reactive protein (mg/dl) | 0.14 (0.05–0.31) |
| Creatinine (mg/dl) | 0.8 (0.7–0.9) |
| ALT (U/l) | 26 (20–35) |
| AST (U/l) | 24 (20–30) |
| Body fat distributionA | |
| Total adipose tissue (TAT, l) | 34.0 (25.6–45.2) |
| Visceral adipose tissue (VAT, l) | 4.5 (3.2–6.5) |
| Subcutaneous abdominal adipose tissue (SCAT, l) | 12.0 (7.9–17.0) |
| Liver fat content (%) | 5.4 (2.4–11.6) |
| GenotypingB | |
| PNPLA3I148MM/MI | 47 (42%) |
| TM6SF2E167KK/KE | 21 (19%) |
| Hemostasis measurements | |
| PT (%) | 89 (80–98) |
| aPTT (sec) | 27 (25–29) |
| Fibrinogen (mg/dl) | 279 (237–315) |
| D-Dimer (μg/ml FEU) | 0.6 (0.4–0.8) |
| Factor II, % | 99 (90–108) |
| Factor VII, % | 95 (76–116) |
| Factor VIII, % | 104 (79–123) |
| Factor IX, % | 103 (89–118) |
| Antithrombin, % | 100 (94–107) |
| Protein C, % | 118 (105–130) |
| Protein S, % | 101 (86–114) |
Data were accessible from the following numbers of participants: A 126, B 112.
Abbreviations: l: liter; OGTT: oral glucose tolerance test; ALT: alanine aminotransferase; PT: prothrombin time; aPTT: activated partial thromboplastin time.
3.2. Relationship between hemostasis parameters, body mass index, and body fat distribution
Linear regression analyses adjusting for age and sex were performed to investigate the relationship between hemostasis parameters, body mass index (BMI), and body fat distribution (detailed results are presented in Table 2). First, we assessed the relation between BMI and hemostasis parameters. BMI was positively associated with fibrinogen, D-Dimer, FVII, FVIII, FIX, and protein S. Antithrombin was negatively associated with BMI.
Table 2.
Relationship between hemostasis parameters, BMI, and body fat distribution.
| BMI, kg/m2 | TAT, l | VAT, l | SCAT, l | Liver fat content, % | |
|---|---|---|---|---|---|
| PT (%) | 0.06 | 0.07 | 0.21∗ | 0.06 | 0.13 |
| aPTT (sec) | −0.16 | −0.11 | −0.21∗ | −0.11 | −0.09 |
| Fibrinogen (mg/dl) | 0.41∗∗∗ | 0.46∗∗∗ | 0.43∗∗∗ | 0.47∗∗∗ | 0.24∗∗ |
| D-Dimer (μg/ml FEU) | 0.26∗∗ | 0.29∗∗ | 0.26∗∗ | 0.27∗∗ | 0.13 |
| Factor II (%) | 0.03 | 0.12 | 0.16 | 0.13 | 0.16 |
| Factor VII (%) | 0.17∗ | 0.10 | 0.20∗ | 0.13 | 0.34∗∗∗ |
| Factor VIII (%) | 0.28∗∗ | 0.28∗∗ | 0.25∗ | 0.25∗∗ | 0.22∗ |
| Factor IX (%) | 0.21∗ | 0.29∗∗ | 0.36∗∗∗ | 0.29∗∗ | 0.14 |
| Antithrombin (%) | −0.34∗∗∗ | −0.33∗∗∗ | −0.30∗∗ | −0.33∗∗∗ | −0.32∗∗∗ |
| Protein C (%) | 0.08 | 0.13 | 0.25∗∗ | 0.14 | 0.32∗∗∗ |
| Protein S (%) | 0.28∗∗∗ | 0.33∗∗∗ | 0.45∗∗∗ | 0.36∗∗∗ | 0.53∗∗∗ |
Given Effect sizes (ßstd) for each association are shown.
Data were adjusted for age and sex.
Asterisks indicate statistical significance: ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Abbreviations: PT: prothrombin time; aPTT: activated partial thromboplastin time; BMI: Body-Mass-Index; TAT: total adipose tissue; VAT: visceral adipose tissue; SCAT: subcutaneous abdominal adipose tissue.
TAT, VAT, and SCAT were positively associated with fibrinogen, D-Dimer, FVIII, FIX, protein S, and negatively with antithrombin. In addition, VAT was positively associated with PT, FVII, protein C, and negatively with aPTT.
Investigating the relationship between liver fat content and hemostasis parameters, a positive association with liver fat content was observed for fibrinogen, FVII, FVIII, protein C, and protein S (results of correlation analyses are given in Figure 1). Again, antithrombin was negatively associated with liver fat content.
3.3. Relationship between hemostasis parameters and metabolism
Next, the relationship between hemostasis parameters and several metabolic variables was investigated. Insulin sensitivity, measured as OGTT-derived insulin sensitivity, was associated with all investigated hemostasis parameters, except PT, FII, and FIX (Table 3). 2 h-glucose was related to FVII and protein C. Furthermore, glycated hemoglobin (HbA1c) was related to aPTT, FVII, and protein C. Finally, C-reactive protein, a marker of inflammation, was found to be associated with all investigated hemostasis parameters, except PT and aPTT.
Table 3.
Relationship between hemostasis parameters and metabolic variables.
| Insulin sensitivity | 2 h-glucose, mmol/l | HbA1c, mmol/mol | CRP, mg/dl | |
|---|---|---|---|---|
| PT (%) | −0.15 | 0.13 | 0.15 | 0.15 |
| aPTT (sec) | 0.21∗ | −0.14 | −0.18∗ | 0.13 |
| Fibrinogen (mg/dl) | −0.23∗∗ | 0.15 | 0.14 | 0.56∗∗∗ |
| D-Dimer (μg/ml FEU) | −0.21∗ | 0.16 | 0.05 | 0.30∗∗∗ |
| Factor II (%) | −0.08 | 0.01 | 0.16 | 0.28∗∗∗ |
| Factor VII (%) | −0.38∗∗∗ | 0.29∗∗∗ | 0.26∗∗ | 0.21∗ |
| Factor VIII (%) | −0.20∗ | 0.15 | 0.05 | 0.36∗∗∗ |
| Factor IX (%) | −0.02 | −0.06 | 0.07 | 0.24∗∗ |
| Antithrombin (%) | 0.31∗∗∗ | −0.10 | −0.02 | −0.18∗ |
| Protein C (%) | −0.24∗∗ | 0.18∗ | 0.20∗ | 0.30∗∗∗ |
| Protein S (%) | −0.37∗∗∗ | 0.06 | 0.10 | 0.35∗∗∗ |
Given Effect sizes (ßstd) for each association are shown.
Data were adjusted for age and sex.
Asterisks indicate statistical significance: ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Abbreviations: PT: prothrombin time; aPTT: activated partial thromboplastin time; CRP: C-reactive protein.
3.4. Differences in hemostasis parameters of subjects with and without fatty liver
To specifically address the impact of increased liver fat on the investigated hemostasis parameters, subjects were divided into two groups comprising subjects with increased liver fat content (>5.56%: “fatty liver”; N = 60) and control subjects (liver fat content ≤5.56%: “controls”; N = 66, table 4). The age and sex of subjects in both groups did not statistically differ. As expected, insulin sensitivity was significantly higher in control subjects, and body weight, BMI, fasting glucose, and total visceral and subcutaneous abdominal fat were significantly higher in subjects with fatty liver (Supplemental Table S2). Searching for differences in the hemostasis system between both groups, fibrinogen (298 mg/dl vs. 264 mg/dl, p = 0.0182), FVII (99% vs. 90%, p = 0.0049), FVIII (114% vs. 90%, p = 0.0098), protein C (124% vs. 111%, p = 0.0006), and protein S (109% vs. 89%, p < 0.0001) increased in subjects with fatty liver (Table 4). Antithrombin (97% vs. 102%, p = 0.0025) was higher in the control subjects. In multiple regression models, FVII, protein C, and protein S remained significantly increased in subjects with fatty liver independent of age, sex, and BMI. Additionally, adjusting for insulin sensitivity, visceral and subcutaneous adipose tissue, and genotype variants (PNPLA3I148MM/MI and TM6SF2E167KK/KE), only protein C and protein S remained significantly increased in subjects with fatty liver.
Table 4.
Comparison of hemostasis parameters between study participants with and without increased liver fat content.
| Controls (N = 66) | Fatty liver (N = 60) | p-value | p-valuea | p-valueb | p-valuec | p-valued | |
|---|---|---|---|---|---|---|---|
| PT (%) | 88 (79–97) | 90 (80–98) | ns | ns | ns | ns | ns |
| aPTT (sec) | 27 (25–29) | 27 (25–29) | ns | ns | ns | ns | ns |
| Fibrinogen (mg/dl) | 264 (228–298) | 298 (253–339) | 0.0182 | ns | ns | ns | ns |
| D-Dimer (μg/ml FEU) | 0.5 (0.4–0.8) | 0.6 (0.3–0.9) | ns | ns | ns | ns | ns |
| Factor II (%) | 97 (89–106) | 100 (94–108) | ns | ns | ns | ns | ns |
| Factor VII (%) | 90 (72–105) | 99 (88–121) | 0.0049 | 0.0169 | ns | ns | ns |
| Factor VIII (%) | 90 (75–116) | 114 (93–131) | 0.0098 | ns | ns | ns | ns |
| Factor IX (%) | 103 (89–117) | 104 (91–119) | ns | ns | ns | ns | ns |
| Antithrombin (%) | 102 (97–109) | 97 (92–103) | 0.0025 | ns | ns | ns | ns |
| Protein C (%) | 111 (101–123) | 124 (113–138) | 0.0006 | 0.0009 | 0.0177 | 0.0209 | 0.0240 |
| Protein S (%) | 89 (78–108) | 109 (98–120) | <0.0001 | 0.0001 | 0.0035 | 0.0036 | 0.0078 |
Results are presented as median and interquartile range (1st – 3rd).
The variation inflation index (VIF) was <3.5 for all analyses.
Abbreviations: PT: prothrombin time; aPTT: activated partial thromboplastin time; ns: not significant; SCAT: subcutaneous abdominal adipose tissue; VAT: visceral adipose tissue.
p-value adjusted for age, sex, and BMI.
p-value adjusted for age, sex, BMI, and insulin sensitivity.
p-value adjusted for age, sex, BMI, insulin sensitivity, SCAT, and VAT.
p-value adjusted for age, sex, BMI, insulin sensitivity, SCAT, VAT, and genotype (PNPLA3I148MM/MI and TM6SF2E167KK/KE).
4. Discussion
The present study aimed to investigate the impact of visceral and subcutaneous abdominal adipose tissue and liver fat content on alterations in the plasmatic coagulation system in patients at risk for type 2 diabetes.
First, we demonstrated that both visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SCAT) along with liver fat are significantly associated with alterations in the plasmatic coagulation system (refer Figure 2). We identified procoagulant factors (FVII, FVIII, and FIX) and anticoagulant proteins (antithrombin, protein C, and protein S) to be associated with VAT, SCAT, and/or liver fat. Most of the differences in plasmatic coagulation between patients with and without fatty liver were driven by important confounders, whereas higher protein C and protein S in the case of fatty liver were independent of those. Our findings indicate that body fat distribution is an important determinant of the alterations in the hemostasis system. In particular, liver fat was identified as an independent determinant of protein C and protein S in subjects at increased risk for type 2 diabetes. These findings suggest a potential counteracting mechanism to the prothrombotic tendency in fatty liver.
Abnormalities in the hemostasis system predisposing to a prothrombotic state are linked to obesity and insulin resistance [8]. In particular, body fat distribution is an established cardiometabolic risk factor explaining the increased risk for cardiovascular diseases in obesity [20]. In the present study, VAT and SCAT were associated with increased activities of several procoagulant factors (FVII, FVIII, and FIX), of which FVIII and FIX are established risk factors for arterial and venous thrombosis [26,27]. The link between visceral (abdominal) obesity and hemostatic alterations is well established and several reports have consistently denoted that increased activities of procoagulant factors are associated with abdominal obesity that agree with the current results [8,17,18]. Our study extends these findings and demonstrates that SCAT is also, to a similar degree as visceral obesity, associated with increased procoagulant factors (FVIII and FIX). In a study by Winfield et al. it was even depicted that SCAT has a stronger relationship with hypercoagulability than VAT, following trauma injury [28]. Consequently, fat accumulation in the two major compartments, visceral and subcutaneous abdominal adipose tissue, contributes to cardiometabolic risk through altered plasmatic hemostasis. In accordance with these findings, antithrombin was negatively associated with VAT and SCAT in the present study. However, the anticoagulant proteins, protein C and protein S, were found to be positively associated with VAT; and protein S was also associated with SCAT. Only a few studies addressed the association of anticoagulant proteins and visceral obesity and found comparable or slightly increased activities of anticoagulant proteins in subjects with visceral obesity compared to control subjects [17,29,30]. To the best of our knowledge, the association of anticoagulant proteins and SCAT has not been studied so far. Our findings support the central role of VAT and SCAT as contributors to the alterations of procoagulant factors and anticoagulant proteins. Hence, fat accumulation in these compartments affects the complex regulation of the hemostasis system involving procoagulant and anticoagulant parameters.
Because most of the investigated hemostasis parameters are mainly produced by the liver, and fatty liver is closely linked to obesity and insulin resistance, the specific role of increased liver fat on hemostasis parameters needs to be elucidated [31]. Hemostatic alterations are known to increase the risk for cardiovascular diseases in nonalcoholic fatty liver disease (NAFLD) [32,33]. Accordingly, we found increased levels of hemostasis parameters in subjects with fatty liver compared to control subjects without fatty liver. However, for most of these parameters, the differences disappeared after adjusting for important confounders. Only protein C and proteins S remained significantly increased in subjects with fatty liver.
Protein C and protein S are vitamin K-dependent proteins and belong to the anticoagulant system. Activated protein C together with protein S can specifically inactivate FVa and FVIIIa, thereby limiting the coagulation process. Increased activities of protein C and protein S have been reported in obese patients and it is speculated that this might represent a compensatory mechanism for the hypercoagulable state in obesity [[34], [35], [36]]. However, antithrombin, another natural anticoagulant, was negatively associated with liver fat which corresponds to the prothrombotic state in participants with fatty liver. NAFLD is considered to be associated with hypercoagulability [37,38], and hence, further studies are required to investigate whether increased activities of protein C and protein S, as observed in our study, can counteract this hypercoagulable state. The increases in protein C and protein S are a specific feature of liver steatosis/NAFLD and cannot be transferred to advanced liver diseases, as patients with liver cirrhosis have markedly decreased levels of anticoagulant proteins including protein C and protein S [37,39].
Few other studies have investigated the role of liver steatosis/fatty liver or liver diseases in relation to single parameters of the hemostasis system and results were often limited to a small number of subjects [37,[39], [40], [41], [42]]. Assy et al. reported increased levels of protein C and protein S in subjects with fatty liver compared to control subjects and patients with chronic hepatitis [39,43]. However, the number of patients with fatty liver was low (N = 10–15) and no adjustments for metabolic variables were made. In a study by Papatheodoridis et al. protein C and protein S levels were reported to be higher in patients with NAFLD (N = 60) compared with chronic viral hepatitis patients (N = 90) [41]. Patients without liver diseases were not included and results were not adjusted for metabolic variables. In our study, we included subjects with an increased risk for type 2 diabetes without overt liver diseases. We performed a comprehensive analysis of procoagulant and anticoagulant parameters in metabolically well-characterized participants using state-of-the-art methods for hemostasis measurements and for the determination of adipose tissue compartments and liver fat content. Results were adjusted for relevant variables including insulin sensitivity, body fat composition, and genotype variants of important regulators of hepatic triglyceride content (PNPLA3I148MM/MI and TM6SF2E167 kK/kE) [44,45]. Therefore, we believe that our analyses may adequately assess the specific role of liver fat on the investigated hemostasis parameters.
In conclusion, our analyses identified visceral and subcutaneous abdominal adipose tissue as potential contributors to the alterations in the hemostasis system. Liver fat was independently associated with protein C and protein S suggesting a potential counteracting mechanism to the prothrombotic tendency in subjects with prediabetes and fatty liver.
Data availability statement
The datasets analyzed during the current study are not publicly available owing to ethical regulations, but are available from the corresponding author on reasonable request.
Funding
The study was supported in part by a grant from the German Center for Diabetes Research (DZD) that is funded by the German Federal Ministry for Education and Research (01GI0925).
Author contributions
Sebastian Hörber: conceptualization, formal analysis, investigation, writing – original draft; Rainer Lehmann: resources, writing - review & editing; Norbert Stefan: formal analysis, writing - review & editing; Jürgen Machann: resources, formal analysis, writing - review & editing; Andreas Birkenfeld: resources, writing - review & editing, Robert Wagner: formal analysis, resources, writing - review & editing; Martin Heni: formal analysis, writing – original draft; Hans-Ulrich Häring: resources, writing - review & editing; Andreas Fritsche: conceptualization, writing – review & editing, Andreas Peter: conceptualization, resources, investigation, writing – original draft, supervision.
Acknowledgments
We gratefully thank all the study participants and acknowledge the technical assistance of Susanne Faix, Janina Roche, Ann Kathrin Horlacher, and Isolde Riedlinger. We thank Jennifer Kriebel and Harald Grallert (Institute of Epidemiology, Molecular Epidemiology, Helmholtz Center Munich) for generating and providing the Global Screening Array data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101262.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Stefan N., Birkenfeld A.L., Schulze M.B. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nature Reviews Endocrinology. 2021;17(3):135–149. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 2.Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nature Reviews Endocrinology. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxford A.E., Halla F., Robertson E.B., Morrison B.E. Endothelial cell contributions to COVID-19. Pathogens. 2020;9(10) doi: 10.3390/pathogens9100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvei L.D., Grimnes G., Hindberg K., Mathiesen E.B., Njolstad I., Wilsgaard T. C-reactive protein, obesity, and the risk for arterial and venous thrombosis. Journal of Thrombosis and Haemostasis. 2016;14(8):1561–1571. doi: 10.1111/jth.13369. [DOI] [PubMed] [Google Scholar]
- 8.Morange P.E., Alessi M.C. Thrombosis in central obesity and metabolic syndrome: mechanisms and epidemiology. Thrombosis & Haemostasis. 2013;110(4):669–680. doi: 10.1160/TH13-01-0075. [DOI] [PubMed] [Google Scholar]
- 9.Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 10.Mina A., Favaloro E.J., Koutts J. Hemostatic dysfunction associated with endocrine disorders as a major risk factor and cause of human morbidity and mortality: a comprehensive meta-review. Seminars in Thrombosis and Hemostasis. 2007;33(8):798–809. doi: 10.1055/s-2007-1000372. [DOI] [PubMed] [Google Scholar]
- 11.Neeland I.J., Ross R., Despres J.P., Matsuzawa Y., Yamashita S., Shai I. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 12.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 13.Petersen A., Bressem K., Albrecht J., Thiess H.M., Vahldiek J., Hamm B. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre G., Legueult K., Pradier C., Raffaelli C., Ichai C., Iannelli A. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115:154440. doi: 10.1016/j.metabol.2020.154440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk for incident cardiovascular disease: a meta-analysis. Journal of Hepatology. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Horber S., Lehmann R., Fritsche L., Machann J., Birkenfeld A.L., Haring H.U. Lifestyle intervention improves prothrombotic coagulation profile in individuals at high-risk for type 2 diabetes. The Journal of Cinical Endocrinology and Metabolism. 2021 doi: 10.1210/clinem/dgab124. [DOI] [PubMed] [Google Scholar]
- 17.Targher G., Zoppini G., Moghetti P., Day C.P. Disorders of coagulation and hemostasis in abdominal obesity: emerging role of fatty liver. Seminars in Thrombosis and Hemostasis. 2010;36(1):41–48. doi: 10.1055/s-0030-1248723. [DOI] [PubMed] [Google Scholar]
- 18.Vilahur G., Ben-Aicha S., Badimon L. New insights into the role of adipose tissue in thrombosis. Cardiovascular Research. 2017;113(9):1046–1054. doi: 10.1093/cvr/cvx086. [DOI] [PubMed] [Google Scholar]
- 19.Stefan N., Fritsche A., Schick F., Haring H.U. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. 2016;4(9):789–798. doi: 10.1016/S2213-8587(16)00082-6. [DOI] [PubMed] [Google Scholar]
- 20.Morelli M., Gaggini M., Daniele G., Marraccini P., Sicari R., Gastaldelli A. Ectopic fat: the true culprit linking obesity and cardiovascular disease? Thrombosis & Haemostasis. 2013;110(4):651–660. doi: 10.1160/TH13-04-0285. [DOI] [PubMed] [Google Scholar]
- 21.Fritsche A., Wagner R., Heni M., Kantartzis K., Machann J., Schick F. 2021. Risk-stratified lifestyle intervention to prevent type 2 diabetes. medRxiv:2021.2001.2026.21249582. [Google Scholar]
- 22.Wurslin C., Machann J., Rempp H., Claussen C., Yang B., Schick F. Topography mapping of whole body adipose tissue using A fully automated and standardized procedure. Journal of Magnetic Resonance Imaging. 2010;31(2):430–439. doi: 10.1002/jmri.22036. [DOI] [PubMed] [Google Scholar]
- 23.Machann J., Thamer C., Schnoedt B., Haap M., Haring H.U., Claussen C.D. Standardized assessment of whole body adipose tissue topography by MRI. Journal of Magnetic Resonance Imaging. 2005;21(4):455–462. doi: 10.1002/jmri.20292. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. American Journal of Physiology. Endocrinology and Metabolism. 2005;288(2):E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 26.van Hylckama Vlieg A., van der Linden I.K., Bertina R.M., Rosendaal F.R. High levels of factor IX increase the risk for venous thrombosis. Blood. 2000;95(12):3678–3682. [PubMed] [Google Scholar]
- 27.Koster T., Blann A.D., Briet E., Vandenbroucke J.P., Rosendaal F.R. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345(8943):152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 28.Winfield R.D., Mellnick V.M., Chamieh J., Nohra E., Tan W.H., Ramirez R. Adipose tissue location and contribution to postinjury hypercoagulability. J Trauma Acute Care Surg. 2016;81(1):79–85. doi: 10.1097/TA.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 29.Bowles L.K., Cooper J.A., Howarth D.J., Miller G.J., MacCallum P.K. Associations of haemostatic variables with body mass index: a community-based study. Blood Coagulation and Fibrinolysis. 2003;14(6):569–573. doi: 10.1097/00001721-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Sola E., Navarro S., Medina P., Vaya A., Estelles A., Hernandez-Mijares A. Activated protein C levels in obesity and weight loss influence. Thrombosis Research. 2009;123(5):697–700. doi: 10.1016/j.thromres.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 32.Lonardo A., Sookoian S., Pirola C.J., Targher G. Non-alcoholic fatty liver disease and risk for cardiovascular disease. Metabolism. 2016;65(8):1136–1150. doi: 10.1016/j.metabol.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Targher G., Byrne C.D., Tilg H. NAFLD and increased risk for cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 34.Agewall S., Bokemark L., Wikstrand J., Lindahl A., Fagerberg B. Insulin sensitivity and hemostatic factors in clinically healthy 58-year-old men. Thrombosis & Haemostasis. 2000;84(4):571–575. [PubMed] [Google Scholar]
- 35.De Pergola G., Pannacciulli N. Coagulation and fibrinolysis abnormalities in obesity. Journal of Endocrinological Investigation. 2002;25(10):899–904. doi: 10.1007/BF03344054. [DOI] [PubMed] [Google Scholar]
- 36.Rega-Kaun G., Kaun C., Ebenbauer B., Jaegersberger G., Prager M., Wojta J. Bariatric surgery in morbidly obese individuals affects plasma levels of protein C and thrombomodulin. Journal of Thrombosis and Thrombolysis. 2019;47(1):51–56. doi: 10.1007/s11239-018-1744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tripodi A., Fracanzani A.L., Primignani M., Chantarangkul V., Clerici M., Mannucci P.M. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2014;61(1):148–154. doi: 10.1016/j.jhep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Spinosa M., Stine J.G. Nonalcoholic fatty liver disease-evidence for a thrombophilic state? Current Pharmaceutical Design. 2020;26(10):1036–1044. doi: 10.2174/1381612826666200131101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assy N., Bekirov I., Mejritsky Y., Solomon L., Szvalb S., Hussein O. Association between thrombotic risk factors and extent of fibrosis in patients with non-alcoholic fatty liver diseases. World Journal of Gastroenterology. 2005;11(37):5834–5839. doi: 10.3748/wjg.v11.i37.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verrijken A., Francque S., Mertens I., Prawitt J., Caron S., Hubens G. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59(1):121–129. doi: 10.1002/hep.26510. [DOI] [PubMed] [Google Scholar]
- 41.Papatheodoridis G.V., Chrysanthos N., Cholongitas E., Pavlou E., Apergis G., Tiniakos D.G. Thrombotic risk factors and liver histologic lesions in non-alcoholic fatty liver disease. Journal of Hepatology. 2009;51(5):931–938. doi: 10.1016/j.jhep.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Kotronen A., Joutsi-Korhonen L., Sevastianova K., Bergholm R., Hakkarainen A., Pietilainen K.H. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver International. 2011;31(2):176–183. doi: 10.1111/j.1478-3231.2010.02375.x. [DOI] [PubMed] [Google Scholar]
- 43.Assy N., Schlesinger S., Hussein O. Elevated plasma protein C levels correlate with the presence of fatty liver (NASH and NAFLD) Gut. 2005;54(5):729. doi: 10.1136/gut.2004.060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjaerg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available owing to ethical regulations, but are available from the corresponding author on reasonable request.