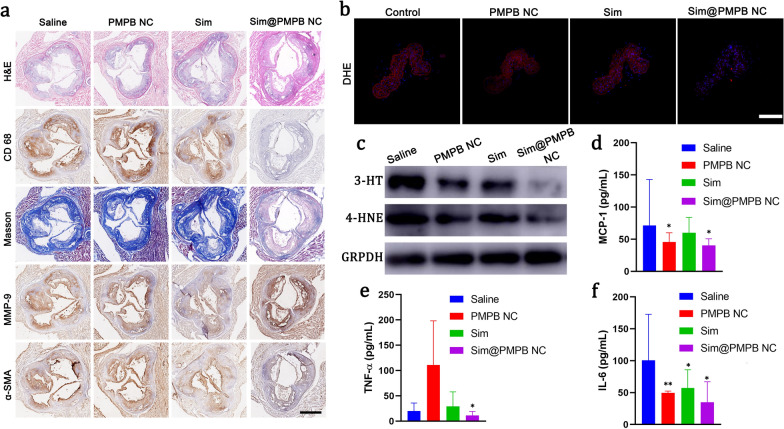
Fig. 5.
Pathological examinations and mechanistic explorations of Sim@PMPB NC treatment through analyzing oxidative stress and inflammation in ApoE−/− mice. a Representative immunohistochemical images of aortic root sections stained with H&E, CD68, MMP-9, Masson's trichrome, and α-SMA, and they were harvested from ApoE−/− mice that experienced a cholesterol-rich and high-fat diet for 3 months and different treatments (once a week) within the last two months. b Fluorescence images of DHE-stained sections of brachiocephalic artery after different treatments (control, PMPB NC, Sim, Sim@PMPB NC). c–e The levels of MCP-1 (c), IL-6 (d) and TNF-α (e) in serum of ApoE−/− mice after different treatments (control, PMPB NC, Sim, Sim@PMPB NC). f Western blotting bands of the representative expressions of 3-NT and 4-HNE. ApoE−/− mice were fed a cholesterol-rich and high-fat diet for 4 months. After the first month, different treatments were performed once a week within the last three months. Mice in the saline group were treated with saline alone, while other groups were separately administered with PMPB NC (20 mg/kg), Sim (2 mg/kg), and Sim@PMPB NC (20 mg/kg). Data are expressed as mean ± SD (n = 7), and *P˂0.05 and **P˂0.01, which were obtained in comparison to Saline. Anti-oxidant drug-loaded prussian blue analogues have been constructed to boycott atherosclerosis via scavenging reactive oxygen species, mitigating inflammation, decreasing collagen accumulation, reducing fibrous cap thickness, hampering macrophage infiltration, blockading foam cell birth and inhibiting lipid protein internalization and eventually depleting plaques.