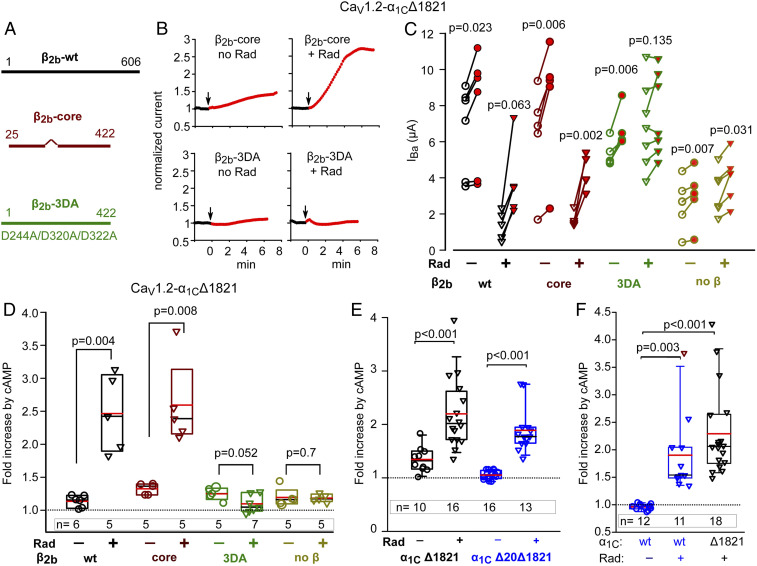
Fig. 2.
Separation of Rad-dependent and Rad-independent PKA regulation of α1C. In all experiments, Rad:β2b RNA ratio was 1:2 or 1:1. (A) Schematic representation of CaVβ variants used. The wild-type (β2b-wt) protein is 606 a.a. long. β-core was truncated at a.a. 422; the linker a.a. 138 to 202 were removed (38). The β2b-3DA is the β2b truncated at a.a. 422, with three Asp-to-Ala mutations, D244A/D320A/D322A. (B–D) the presence of the β subunit and its ability to bind Rad are crucial for Rad-dependent but not for Rad-independent cAMP regulation of CaV1.2. 1 experiment. (B) Diary plots of cAMP-induced changes in IBa (see SI Appendix, Fig. S1 for additional examples). (C) Before–after plots of cAMP-induced changes in IBa. Statistics: paired t test. (D) Summary of data from C. Data show the fold increase with Rad coexpression (inverted triangles) and without Rad (circles). Groups with and without Rad were compared by Mann–Whitney U Rank Sum test (t test for β2b-3DA groups in which normality was satisfied). (E) The role of N-terminal initial segment of α1C. Data shown are cAMP-induced changes in IBa in individual cells expressing CaV1.2-α1CΔ1821 (black) and CaV1.2-α1CΔ20Δ1821 (red; the latter is lacking the first 20 a.a. of the N terminus), with α2δ and β2b, without or with Rad. Refer to SI Appendix, Fig. S1B for raw data. Three experiments; statistics: Mann–Whitney U test. (F) The role of dCT of α1C. Data show the fold increase in IBa after cAMP injection (raw data are shown in SI Appendix, Fig. S1 C and D). Cells expressed the full length α1C (α1Cwt) or α1CΔ1821, α2δ and β2b, without or with Rad. Three experiments; statistics: Kruskal–Wallis test; H = 27.017 with 2 degrees of freedom, P = <0.001.