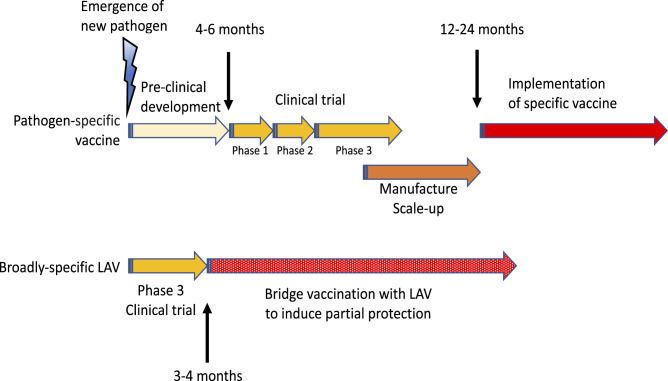
Fig. 3.
Specific vaccines against a new pathogen require extensive preclinical and clinical development and scaling up their production. Because of the urgency, some of the steps can be combined (e.g., phases 1 and 2, or 2 and 3 of clinical trials). On the other hand the existing LAVs only need phase 3 trial of their efficacy. They can either be stockpiled in advance or their manufacture could be ramped up in parallel with testing. Therefore, LAVs can be deployed sooner and used for bridge vaccination to provide (partial) protection and limit the spread of the pandemics until the development of specific vaccines.