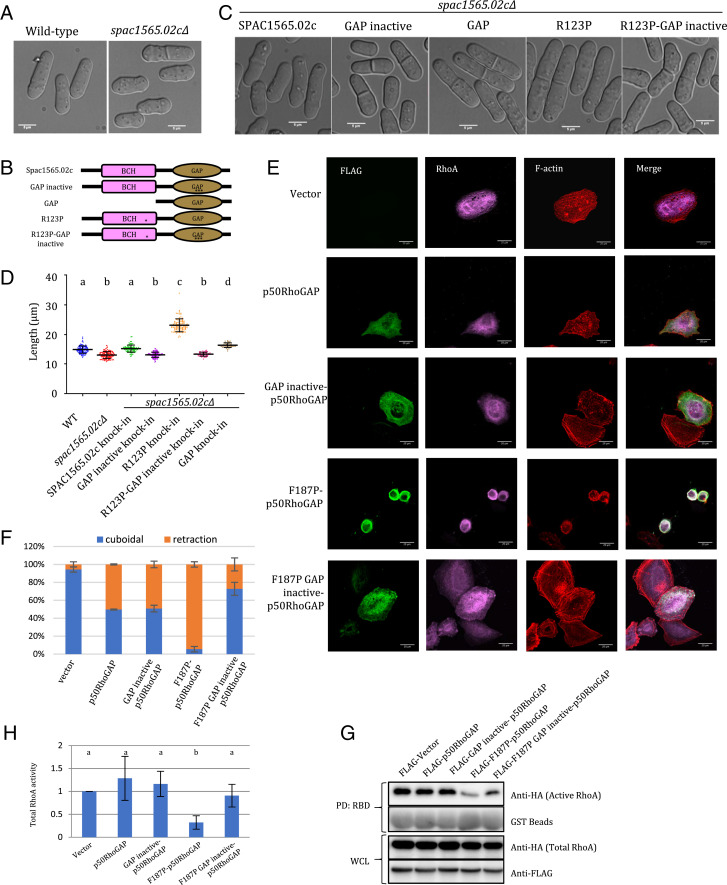
Fig. 4.
Disruption of the β5 via R123P (YBCH) or F187P (HBCH) mutation activates GAP activity of Spac1565.02c (yeast p50RhoGAP) and p50RhoGAP, respectively. (A) Differential interference contrast images of WT (n = 107) and spac1565.02cΔ (n = 124) S. pombe cells (measurements from three independent experiments). spac1565.02cΔ cells were 12% shorter than WT cells. (B) Pictorial representation of various SPAC1565.02c constructs as indicated in B. * represents R123P, and *** represents Arg finger mutation (R200A/K201A/N308A). (C) spac1565.02cΔ cells with knocked-in constructs containing SPAC1565.02c (n = 93), GAP inactive (n = 116), GAP alone (n = 47), R123P (n =100), or R123P-GAP inactive (n = 57). (D) Dot plot of the length of the different yeast cell populations as described in A and C. Measurements from three independent experiments. Different letter denotes statistical significance at P < 0.05. Data was plotted and analyzed by Prism, Graphpad. (E) HeLa cells were cotransfected with HA-RhoA and FLAG-p50RhoGAP or its mutants as labeled. Immunostaining was performed. Representative images are shown. (Scale bar, 20 μm.) (F) The ratios of cuboidal and round cells were scored with at least 100 transfected cells counted per sample per experiment. The scores for “shrinkage” and “rounded” cells as “retraction” group. Data are means ± SD (n = 3). Scoring of the transfected cells were based on the criteria set by the study of Zhou et al. (13). (G) 293T cells were transfected with the expression vectors HA-RhoA and the FLAG-p50RhoGAP or its mutants as indicated. Cell lysates were incubated with immobilized GST-Rho–binding domain of rhotekin. Bound active RhoA were resolved by SDS-PAGE and detected by anti-HA antibody. Whole-cell lysate indicates equal loading. (H) Densitometry analysis of the active RhoA (RhoA-GTP bound by RBD assay) with the vector set as reference. Error bars represent SDs. Different letters denote statistical significance at P < 0.05. n = 5. Student’s t test was performed.