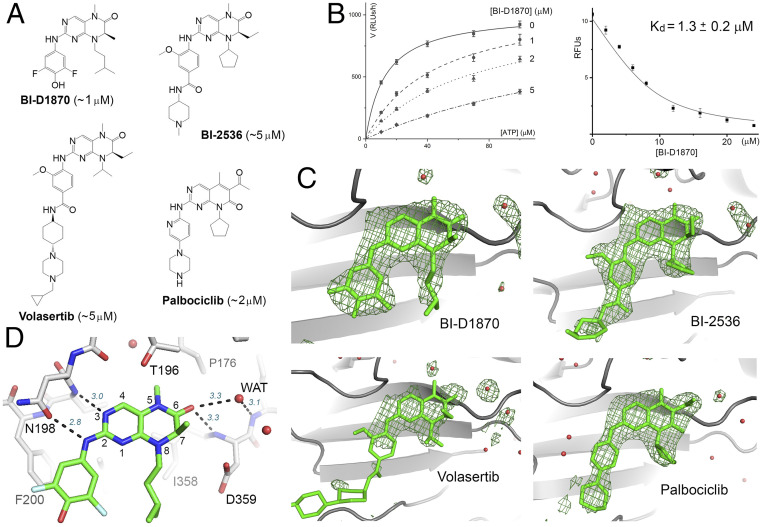
Fig. 1.
Hit molecules with a 2-amino-dihydropteridinone core. (A) The chemical structures of the hit molecules and their Kis for PI5P4Kα (shown in the parentheses). The core structure of palbociclib is slightly different but engages in similar hydrogen-bonding interactions with the kinase. (B) Michaelis–Menten curves obtained at different BI-D1870 concentrations (based on ADP-Glo measurement of relative luminescence unit, or RLU; Left). BI-D1870 displaces a bound fluorescent ATP analog from PI5P4Kα, which provides an independent measurement of its Kd (RFU, relative fluorescence unit; Right) (66). (C) Difference Fourier analysis confirms inhibitor binding to the ATP-binding pocket of PI5P4Kα. Fo-Fc maps, contoured at 3σ, are shown in green. Red spheres represent water. The cyclohexane piperazine side chain of volasertib is largely disordered. (D) Key binding interactions exemplified by the crystal structure of PI5P4Kα in complex with BI-D1870. Hydrogen bonds are shown as dashed lines with distance labeled (italic). The numbering system for pteridine is also shown.