Figure 1.
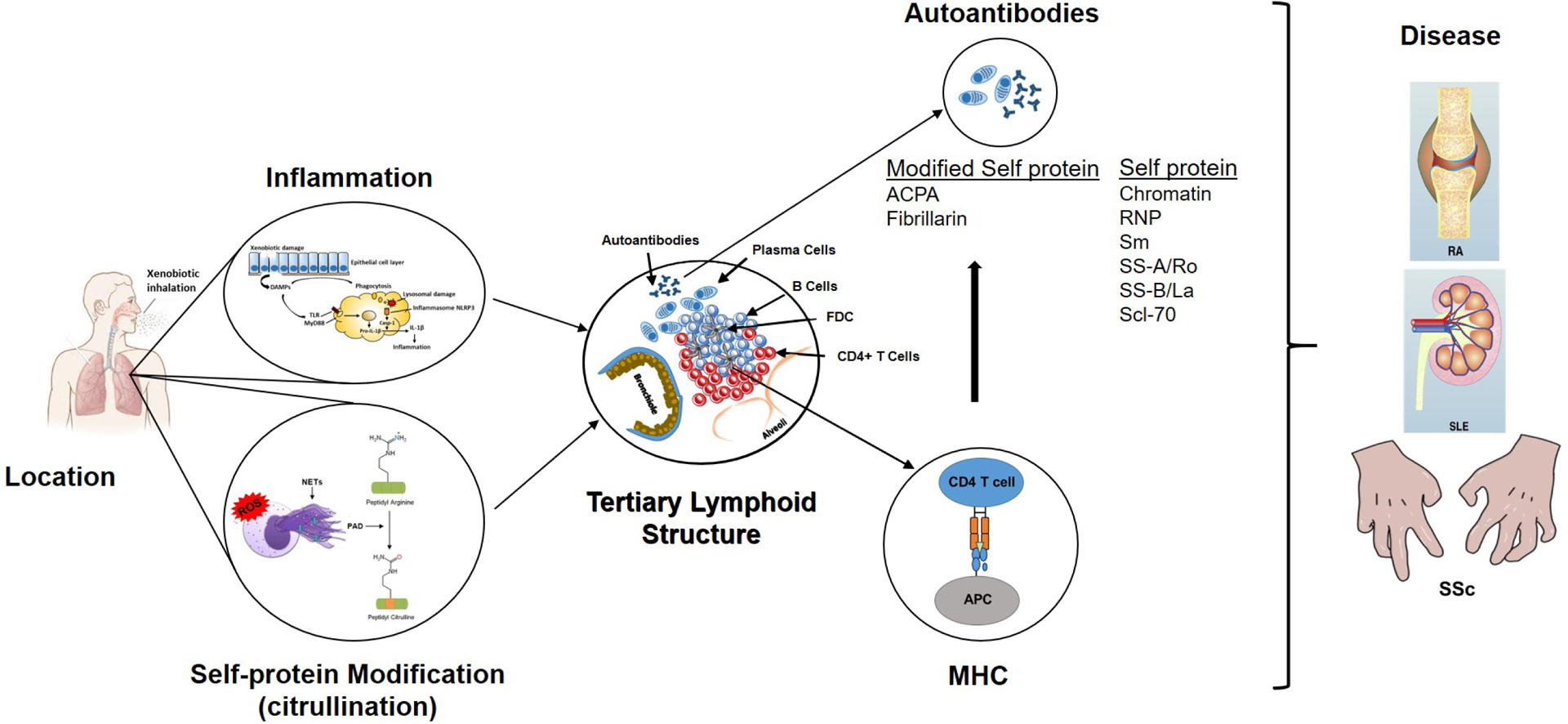
Steps in the development of xenobiotic-induced autoimmunity and autoimmune disease. Information in the figure summarizes the major points discussed in the text as they relate to the seven hypothesized steps leading to autoimmunity or autoimmune disease following xenobiotic exposure. The lungs serve as a common site of xenobiotic exposure. Chronic exposure results in an inflammatory response beginning with cellular and tissue damage, DAMP activation of PRR including TLRs and expression of inflammatory cytokines. Phagocytosis of DAMPS, including particulate xenobiotics, leads to lysosomal damage, inflammasome activation and processing of pro-IL-1β, further enhancing inflammation. Coincident with the inflammatory response are stress response related events, including NETosis and release of PAD enzymes, that leads to post-translational modification of self-proteins, particularly citrullination, and production of neoantigens. The ensuing chronic inflammatory response results in development of TLS comprised of accumulations of B cells within surrounding CD4+ T cells, FDCs and plasma cells. Processing and presentation of self and modified-self proteins, particularly in the context of MHC restriction, leads to autoantibodies. Under appropriate gene-environment interactions these autoantibodies can have diagnostic specificity such as the ACPA response in RA. The culmination of these steps is the development of disease pathology in distant tissues such as the joint in RA, the kidney in SLE and sclerodactyly, a skin manifestation of SSc.
