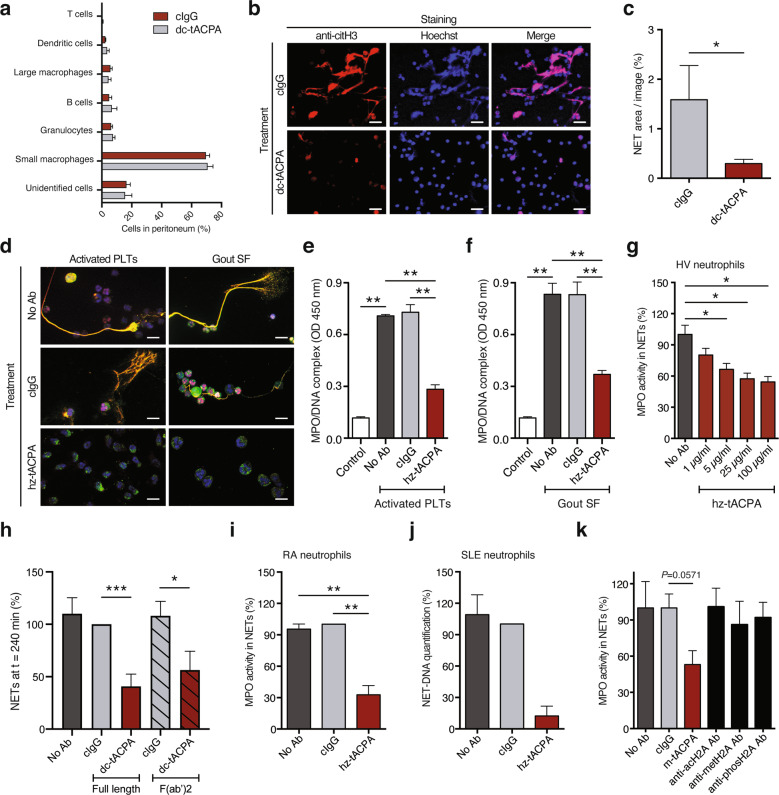
Fig. 5.
tACPA inhibits NET formation in vivo and in vitro. To induce inflammation in the peritoneum, pristane was injected, followed by cIgG or dc-tACPA. a The composition of peritoneal cell infiltrates was analyzed after 24 h (n = 7–8). b Representative images showing NET formation in the peritoneum of mice treated with cIgG or dc-tACPA. NETs were stained with Hoechst (blue) and anti-citH3 antibody (red). Scale bars: 50 µm. c Quantification of NETs (colocalization of citH3 and Hoechst; n = 10). d Representative images showing in vitro human NET release induced by activated PLTs or gout SF without antibody (No Ab) or in the presence of cIgG or hz-tACPA. Scale bars: 20 µm, bottom right image: 10 µm. NETs were stained with DAPI (blue), anti-citH3 antibody (red), and anti-NE antibody (green). MPO/DNA complexes were measured in harvested NETs upon stimulation with activated e PLTs or f gout SF (n = 5). g The activity of MPO present in NETs of neutrophils from HVs was measured after A23187 stimulation in the presence of different concentrations of hz-tACPA (n = 4). h The percentage of NETs at t = 240 min in A23187-stimulated neutrophils from HVs that were treated with either full-length dc-tACPA or dc-tACPA F(ab′)2 antibody fragments was quantified (n = 8). MPO activity in NETs or NET-DNA was quantified in A23187-stimulated neutrophils from i RA and j SLE patients (n = 5 and n = 3, respectively) in the absence or presence of cIgG or hz-tACPA. k MPO activity in human A23187-induced NETs without antibodies or with cIgG, m-tACPA, antibodies against acetylated H2A (anti-acH2A), methylated H2A (anti-metH2A), or phosphorylated H2A (anti-phosH2A) was measured (n = 4). To determine the percentage of DNA and MPO activity in NETs, the mean of the No Ab group was set at 100%, and individual percentages were calculated (g and k). In (i) and (j), the mean of the cIgG group was set at 100%. The results are presented as the means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 using two-tailed Mann–Whitney statistical test