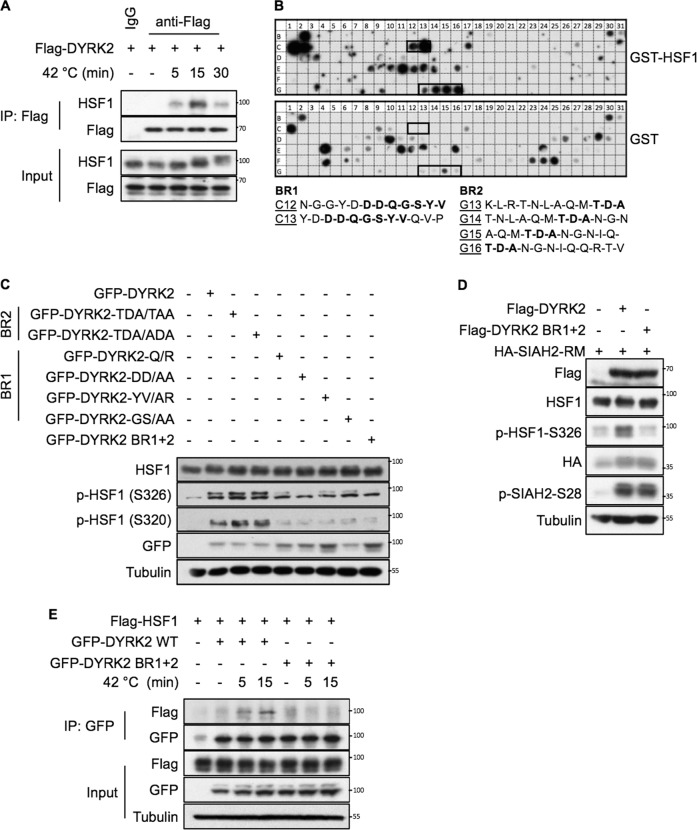
Fig. 2. DYRK2 interacts with HSF1 via two domains.
A 293T cells were transfected with the indicated plasmids. After 48 h, cells were incubated at 42 °C for the indicated periods of time. A fraction of the cell lysates was tested for the correct expression of the transfected DYRK2 (Input), while the remaining extracts were used for immunoprecipitation with either anti-Flag antibodies or with the matched IgG. After elution of bound proteins in 1X SDS sample buffer, coprecipitated HSF1 was visualised by immunoblotting. B A peptide array library covering the complete sequence of DYRK2 was incubated with recombinant GST-HSF1 protein (upper panel) or a GST control protein (lower panel) and the bound HSF1 protein was revealed by immunoblotting against GST as shown. The specific positive binding regions for HSF1 were indicated with a black box. Sequences of DYRK2 peptides within the binding region 1 (BR1) or binding region 2 (BR2) interacting with HSF1 are shown in bold. C 293T cells were transiently transfected with the GFP-tagged versions of either DYRK2-WT or DYRK2 constructs harbouring mutations on the BR1 or BR2 as indicated, or a DYRK2 YV/AR-T/A mutant harbouring mutations in both regions (DYRK2 BR1 + 2). After 48 h, cells were lysed and the levels of endogenous HSF1 and phospho-HSF1 were analysed as indicated. D 293T cells were transiently transfected with the Flag-tagged versions of either DYRK2-WT or DYRK2 BR1 + 2, together with an inactive form of SIAH2 (HA-SIAH2-RM). After 48 h, cells were lysed and the levels of the indicated proteins were analysed as indicated. E 293T cells were transfected with the indicated plasmids. After 48 h, cells were incubated at 42 °C for the indicated periods of time. A fraction of the cell lysates was tested for the correct expression of the transfected proteins (input), while the remaining extracts were used for immunoprecipitation with anti-GFP antibodies. After elution of bound proteins in 1X SDS sample buffer, coprecipitated HSF1 was visualised by immunoblotting using an anti-Flag antibody as shown. See also Fig. S2.