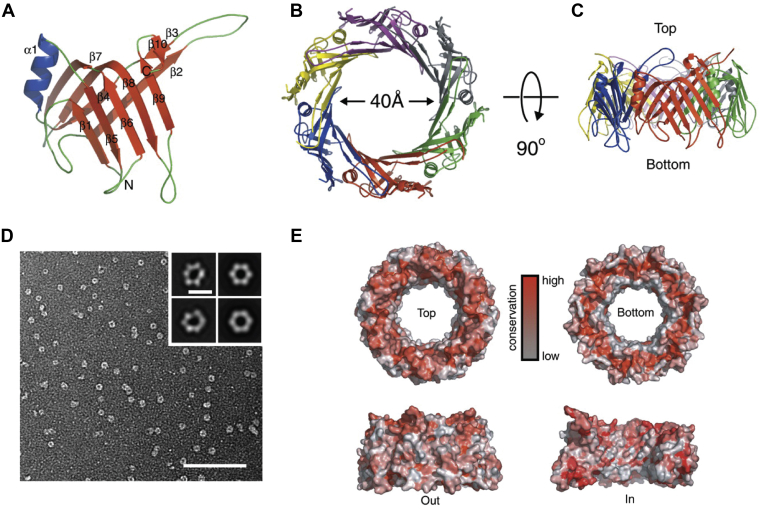
Figure 3.
Structure of Hcp1 protein. Hsp1 forms a hexameric ring with a large internal diameter. A, Ribbon representation of the Hcp1 monomer colored by secondary structure: b strands, red; a helices, blue; and loops, green. B, Top view of a ribbon representation of the crystallographic Hcp1 hexamer. The individual subunits are colored differently to highlight their organization. C, edge-on view of the Hcp1 hexamer shown in (B). D, electron microscopy and single-particle analysis of Hcp1. Electron micrograph of Hcp1 negatively stained with 0.75% (w/v) uranyl formate. Scale bar, 100 nm. Inset, Left, representative class averages and (right) the same averages after 6-fold symmetrization. Inset scale bar, 10 nm. E, sequence conservation analysis of Hcp1. An alignment of 107 Hcp proteins in 43 Gram-negative bacteria was used to plot the relative degree of conservation at each amino acid on the surface of Hcp1. Conservation is indicated by color, where red residues are highly conserved and white residues are poorly conserved. Figure from (35).