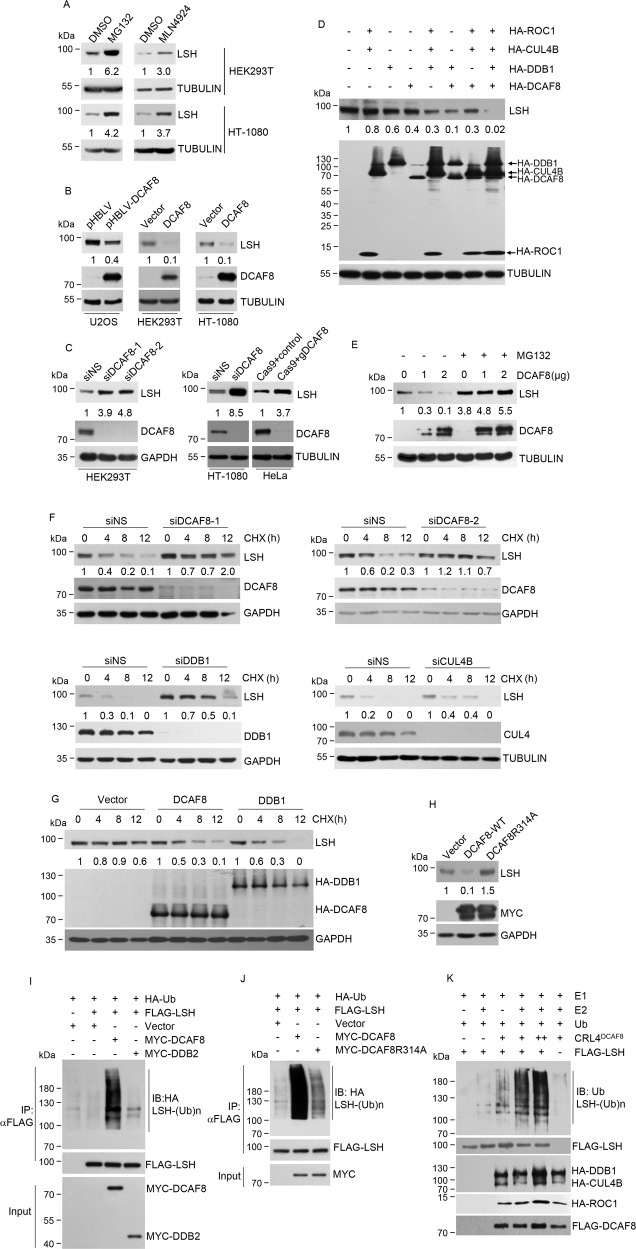
Fig. 2. CRL4DCAF8 is a ubiquitin E3 complex targeting LSH for its proteasomal degradation.
a Western blotting analysis for the indicated proteins from HEK293T and HT-1080 cells treated with MG132 (20 μM, 5 h), MLN4924 (1 μM, 24 h), or DMSO (dimethyl sulfoxide, 1.1 mg/ml) as a control. The relative densities of LSH indicated below the blots were first normalized to that of TUBULIN and then calculated as ratio relative to the value in DMSO-treated cells. b U2OS cells stably transfected with DCAF8 or HEK293T and HT-1080 cells transiently transfected with DCAF8 together with the control cells were collected for western blotting analysis of indicated proteins. The relative densities of LSH indicated below the blots were first normalized to that of TUBULIN and then calculated as ratio relative to the value in control cells. c HEK293T and HT-1080 cells were transfected with control or siRNA against DCAF8. CRISPR-Cas9-mediated DCAF8 ablation was performed in HeLa cells. Protein extracts from these cells were subjected to western blotting analysis with indicated antibodies. siNS, nonspecific RNAi control. The relative densities of LSH indicated below the blots were first normalized to that of TUBULIN or GAPDH and then calculated as ratio relative to the value in control cells. d Individual or combinational transfection of the indicated CRL4DCAF8 complex components was performed in HEK293T cells. Protein extracts from these cells were subjected to western blotting analysis with indicated antibodies. The relative densities of LSH indicated below the blot were first normalized to that of TUBULIN and then calculated as ratio relative to the value in the absence of the four plasmids. e HEK293T cells transfected with DCAF8 were further incubated with MG132 (20 μM) for 5 h. Protein extracts from these cells together with nontreated control cells were subjected to western blotting analysis. The relative densities of LSH shown below the blot were first normalized to that of TUBULIN and then calculated as ratio relative to the value in the absence of MG132 and DCAF8. f HEK293T cells transfected with indicated siRNAs were treated with 40 μg/mL cycloheximide (CHX) for indicated times, and harvested for western blotting analysis. The relative densities of LSH shown were first normalized to that of GAPDH or TUBULIN and then calculated as ratio to the value in the absence of CHX. g HEK293T cells transfected with plasmids expressing indicated proteins were treated with 40 μg/mL cycloheximide for indicated times and harvested for western blotting analysis. The relative densities of LSH shown were first normalized to that of GAPDH and then calculated as ratio to the value in the absence of CHX. h HEK293T cells transfected with control, MYC-tagged wild-type, or R314A mutated DCAF8 were subjected to western blotting analysis with indicated antibodies. The relative densities of LSH indicated below the blot were first normalized to that of GAPDH and then calculated as ratio relative to the value in vector-transfected cells. i 48 h post-transfection with indicated plasmids, HEK293T cells were further treated with MG132 (20 μM) for 5 h. The cells were then collected for immunoprecipitation with anti-FLAG antibody to enrich LSH proteins. Ubiquitination of LSH was then examined by immunoblotting analysis by using of anti-HA antibody. j HEK293T cells transfected with control, wild-type, or mutated DCAF8 together with other indicated plasmids were subjected to LSH ubiquitination analysis as in i. k An in vitro ubiquitination system with purified E1, E2, and ubiquitin proteins and immunopurified core components of CRL4DCAF8 complex and FLAG-LSH from eukaryotic cells.