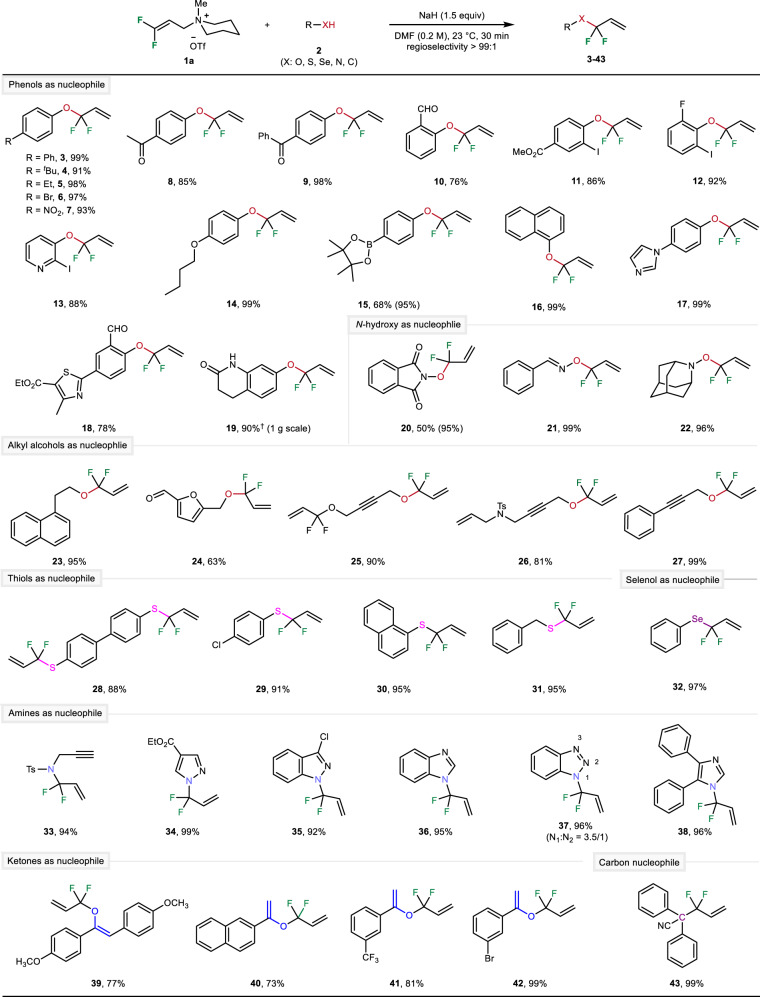
Fig. 3. Regioselective substitution of 1a by different O-, N-, S-, Se- and C-nucleophiles: Substrate scope.
Standard reaction conditions: 1 (1.2 equiv), 2 (1 equiv), NaH (1.5 equiv) in DMF (0.2 M), the reaction mixture was performed at 23 °C under argon atmosphere for 30 min, isolated yield, the α/γ regioselectivity was determined by crude 19F NMR analyses. †Cs2CO3 (0.5 equiv) and toluene (0.1 M) were used as base and solvent, the reaction was performed at 50 °C under air atmosphere for 18 h.