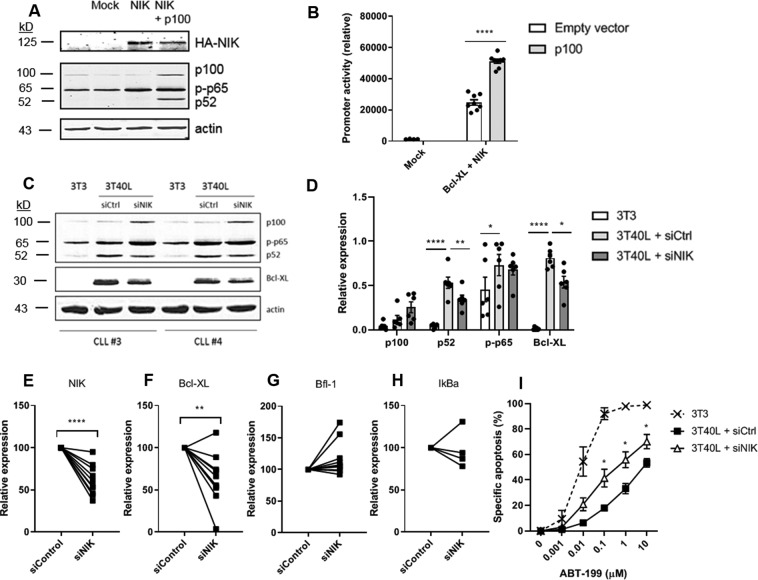
Fig. 4. Non-canonical NF-κB signaling is critical for Bcl-XL expression in CLL.
A HEK293T cells were transfected with empty pGL3-basic vector (mock) or HA-tagged NIK with or without cotransfection of p100. Protein lysates were probed for HA, p100, p-p65, p52, and actin as loading control. B HEK293T cells were transfected with Bcl-XL promoter luciferase reporter gene constructs as well as HA-tagged NIK, with or without cotransfection of p100 (n = 2). Promoter activity was measured and bars represent the mean ± SEM of the luciferase activation normalized to the empty pGL3-basic vector, **p < 0.01 (one-way ANOVA). C CLL cells were nucleofected with either a non-targeting control siRNA (siCtrl) or an siRNA targeting NIK (siNIK) and subsequently cultured on 3T40L for 24 h. After detachment, protein lysates were made and probed for p100, p-p65, p52, Bcl-XL, and actin as loading control. Blots of two representative CLL samples are shown. D Densitometric analysis of p100, p52, p-p65, and Bcl-XL are shown (n = 6). Bars represent the mean ± SEM, *p < 0.05, **p < 0.01, ****p < 0.0001 (two-way ANOVA). E–H CLL cells were nucleofected with NIK siRNA or control siRNA and cultured on 3T40L for 24 h. NIK (n = 10) (E), Bcl-XL (n = 10) (F), Bfl-1 (n = 10) (G), and IκBα (n = 4) (H) mRNA expression was measured by real-time PCR. Samples were normalized to HPRT, **p < 0.01, ****p < 0.0001 (paired sample t-test). I After detachment, cells were incubated with 0.001–10 μM ABT-199 for 24 h after which viability was measured by flow cytometry using DiOC6 and TO-PRO-3 viability dyes (n = 4). Bars represent the mean ± SEM, *p < 0.05 (paired sample t-test (3T40L + siCtrl versus 3T40L + siNIK)).