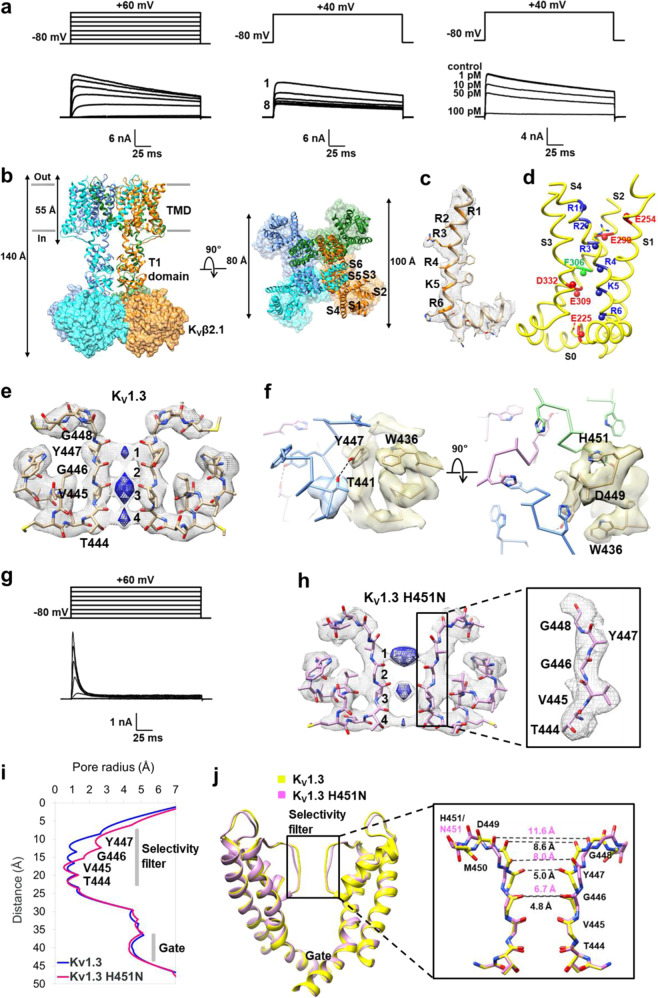
Fig. 1. Cryo-EM structure of human KV1.3–KVβ2.1 channel complex.
a The human KV1.3 channel used in the cryo-EM studies (lacking residues 1–52) exhibits properties similar to the native KV1.3 channel in human T lymphocytes. Voltage-dependent activation (left), use-dependent inactivation (middle), and block by KV1.3-specific inhibitor ShK-EWSS (right). b Model of the hKV1.3–KVβ2.1 complex viewed from the membrane plane (left) and from the extracellular side (right). KV1.3 subunits are represented as a ribbon, and the KVβ2.1 subunits as a surface. c Density for S4 and the S4–S5 linker in KV1.3. Six positively charged residues (R364, R367, R370, R373, K376, and R379) in S4 helix are numbered R1, R2, R3, R4, K5, and R6, respectively. d Structure of the KV1.3 VSD. The α-carbons of negatively charged and positively charged residues are shown as red and blue spheres, respectively. F306 (green) separates negatively charged residues located at the outer and inner ends of the VSD. The α-carbon of R4 (R373) is at the level of F306. e The selectivity filter of KV1.3. The blue densities are shown at a higher contour level (0.017) than the gray ones (0.013). f The key residues, D449, W436, Y447, and T441, which are expected to be involved in C-type inactivation are shown with the corresponding map. Each subunit is shown in stick with a different color. Potential hydrogen bonding interactions between Y447 and T441 are represented as dashed lines. The distance between oxygen atoms in side chains of Y447 and T441 is 3.4 Å. g Voltage-dependent activation of the H451N mutant. h The selectivity filter of KV1.3 H451N.The blue densities are shown at a higher contour level (0.018) than the gray ones (0.014). i Channel pore radius of KV1.3 (blue) and KV1.3 H451N (pink) calculated using the HOLE program. j Structural comparison of the pore-domain in hKV1.3 (yellow) and the H451N mutant (pink). Close view of the selectivity filter and distances between carbonyl oxygen atoms of G446, Y447, and G448 from two diagonally opposed subunits are shown for KV1.3 and KV1.3 H451N, respectively.