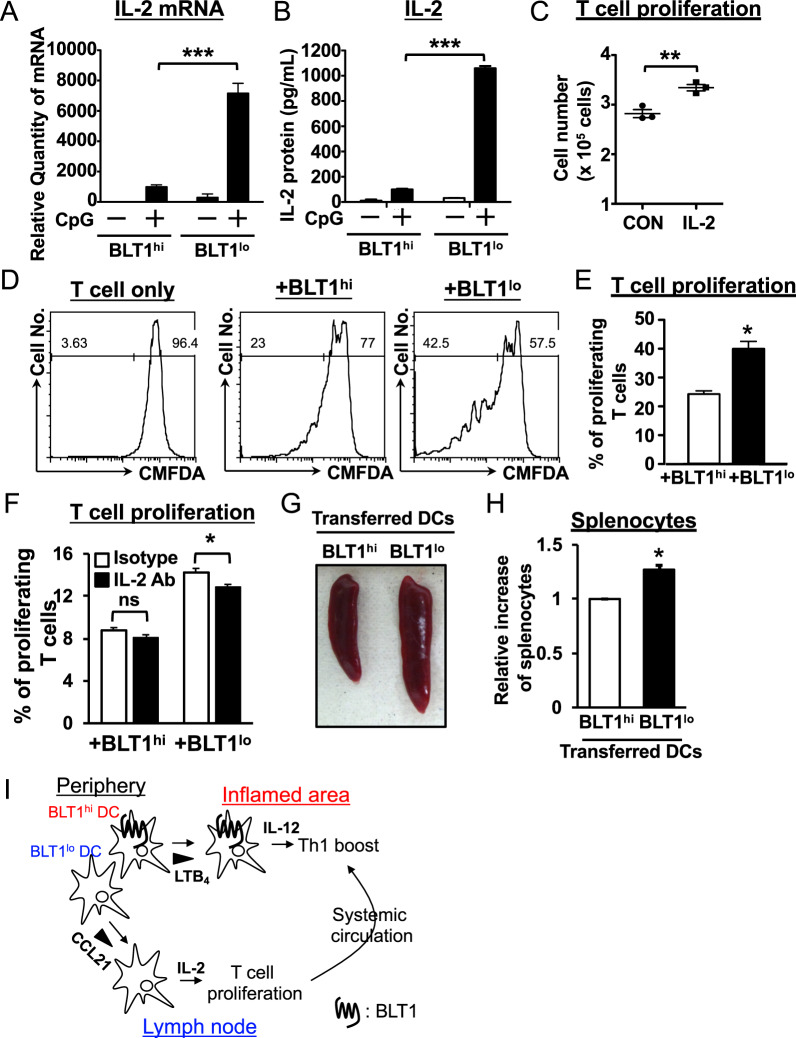
Fig. 6.
BLT1lo DCs preferentially induce T cell proliferation by producing IL-2. a, b BLT1hi and BLT1lo DCs were treated with CpG DNA (500 nM) for 4 h and 24 h, followed by QPCR and CBA analyses for IL-2, respectively. c CD4+ T cells were cultured for 3 days with a recombinant IL-2 protein (10 ng/ml) or vehicle (CON), and cell numbers were counted. CD4+ T cells derived from the OT-II Tg spleen were stained with CMFDA and cocultured for 3 days (d, e) and 2 days (f) with either BLT1hi or BLT1lo DCs in the presence of an OVA peptide. Proliferating CD4+ T cells were analyzed by flow cytometric analysis (n = 3; error bars indicate the S.E.M.). A neutralizing antibody against murine IL-2 was added to the coculture system in f (10 μg/ml). A rat IgG2a kappa isotype control antibody was added to the control group. g, h BLT1hi and BLT1lo DCs were loaded with the OVA peptide (1 μg/ml) for 16 h and injected intravenously into OT-II Tg mice. CpG DNA was also injected intraperitoneally. At three days post-DC transfer, spleen size and splenocyte number were evaluated (n = 2; error bars indicate the S.E.M.). *P < 0.05; **P < 0.01; ***P < 0.001; unpaired Student’s t-test (c, e, g); one-way ANOVA with Bonferroni’s post hoc test (a, b). i The schematic model shows how the novel BLT1hi and BLT1lo DC subsets control skin inflammation. There are two DC subsets, BLT1hi and BLT1lo DCs, in peripheral tissues. BLT1lo DCs migrate preferentially toward draining lymph nodes and produce high amounts of IL-2 to induce T cell proliferation. On the other hand, BLT1hi DCs migrate toward LTB4, which is produced by inflammatory cells, such as neutrophils, in inflammatory areas. BLT1hi DCs produce large amounts of IL-12, which boosts Th1 differentiation. Expanded differentiated Th1 cells produce IFN-γ, which drives spongiosis and edema in inflamed peripheral tissues