Abstract
Sporogenesis is a developmental process that defines embryophytes and involves callose, especially in the production of the highly protective and recalcitrant spore/pollen wall. Until now, hornworts, leptosporangiate ferns and homosporous lycophytes are the only major plant groups in which the involvement of callose in spore development is equivocal. Through aniline blue fluorescence and immunogold labeling in the transmission electron microscope, we provide indisputable evidence for the presence of callose in the spore wall of five hornwort genera, but not in the derived Dendroceros, an epiphyte that produces multicellular spores. We present evidence that callose appears in the developing spore wall and is retained throughout development as a wall constituent of the intine or inner spore wall, a novel location for this polysaccharide in embryophytes. In endosporic and multicellular spores/pollen of Dendroceros, the liverwort Pellia, and Arabidopsis, callose appears in the newly formed cell walls only following the first mitotic division. Further probing for other wall polymers in hornworts reveals the presence of cellulose (Calcofluor fluorescence) in the spore intine, aperture and around the equatorial girdle. Further immunogold labeling with monoclonal antibodies identifies pectin and hemicellulose in hornwort intines. The persistence of callose, a typically transient cell wall constituent, with cellulose, pectins and hemicellulose in the intine, supports specialized functions of callose in spores of hornworts that include reduced water loss when spores are dry and mechanical flexibility to withstand desiccation.
Keywords: bryophyte, callose, cell wall, hornwort, intine, spore
Introduction
Callose is a 1-3-β-glucan cell wall polysaccharide that serves diverse essential functions in plant development, reproduction, and stress responses (Piršelová and Matušíková 2013). Among the key processes that require this wall polymer is sporogenesis, a process that culminates in single-celled meiotic spores that are encased in highly durable sporopollenin-containing walls (Brown et al. 2015). As a signature and diagnostic feature of land plants, spores were instrumental to the successful colonization and radiation of embryophytes nearly 500 million years ago (Renzaglia et al. 2000, 2007; Ligrone et al. 2012). Indeed, thick walled fossil spores (cryptospores) provided the first evidence of plant life on land 40 million years prior to the appearance of plant macrofossils (Brown and Lemmon 2011). In non-seed plants such as bryophytes, spores play a role similar to seeds in that they function as the chief dispersal and perennating structures, remaining dormant for decades as they establish or reestablish populations when habitats become available. Therefore, understanding the intricacies of sporogenesis is important not only in unraveling early plant evolution but also in ascertaining the function of key cell wall polysaccharides across plant groups and over time.
Sporogenesis involves the sequential development of special cell walls that organize and guide the building of one of the most elaborate and structurally unique walls known in the plant life cycle. Spore wall construction begins just prior to or during meiosis when a template for the sculptured spore or pollen wall is organized within the diploid mother cell wall. In many plant groups, including liverworts, heterosporous lycophytes, homosporous ferns and seed plants, this template contains callose (Wallace et al. 2011; Renzaglia et al. 2015). In seed plants, callose has been suggested to restrict the primeexine matrix that is produced by the microspores at the tetrad-stage during development. It is within the primexine that the exine pattern is established and the pollen wall is formed (Dong et al., 2005; Nishikawa et al., 2005; Radja et al. 2019). In liverworts in contrast, sporopollenin is assembled within a callosic template that is replaced progressively as the outer spore wall forms (Renzaglia et al. 2015; Brown and Lemmon 1987). In their review of sporogenesis in bryophytes, Brown and Lemmon 1990, reported an absence of callose in sporogenesis of mosses and hornworts. With more careful examination and higher resolution techniques that include immunogold labeling, Schuette et al. 2009, demonstrated that in mosses callose appears late in spore development and is restricted to the aperture in the nearly mature spore. This was the first description of callose as a component of the moss spore wall. No involvement of callose in the deposition of the spore wall has been identified in mosses. To date, no studies have unequivocally demonstrated the involvement of callose during any portion of hornwort sporogenesis. Although Ridgeway 1965, reported the absence of callose in all stages of sporogenesis in hornworts, Neidhart 1979, noted callose as a component of the spore mother cell wall, but did not provide corroborating evidence.
To investigate whether or not callose is found in any stage of hornwort spore development, we conducted a correlated microscopic investigation on sporogenesis in Phaeoceros carolinianus (Michx.) Prosk and Notothylas orbicularis (Schwein.) Sull. ex A. Gray. The entire process of sporogenesis was followed using aniline blue staining with fluorescence microscopy and immunogold labeling in the transmission electron microscope. Following positive identification of callose in the intine, or endospore, of these plants, we probed four additional hornwort genera (Leiosporoceros, Anthoceros, Megaceros and Dendroceros), representing the entire diversity within the group. To assess if other wall polymers co-localized with callose, we probed for pectins and hemicellulose in two taxa: Phaeoceros and Leiosporoceros. The precocious, endosporic spores of Dendroceros lack callose in the spore wall when single-celled but contain this polymer in newly developed walls of multicellular spores. To determine if callose is present in other endosporic taxa, we probed multicellular spores/ pollen of Pellia (liverwort) and Arabidopsis and found that callose does not occur in the mature intine but occurs only in newly laid down cell walls following mitosis.
Materials and Methods
Plant Species and collections
Arabidopsis thaliana is from culture of Donor Stock Number CS1601 located at the Arabidopsis Biological Resource Center. The species and authorities, locations and collections for field collected plants are listed below:
Leiosporoceros dussii (Steph.) Hässel, PANAMA, Villarreal 803a, Anthoceros agrestis Paton nom. cons. prop., ILLINOIS, Renzaglia 4146, Phaeoceros carolinianus (Michx.) Prosk., ILLINOIS, Renzaglia 4139, Notothylas orbicularis (Schwein.) Sull., ILLINOIS, Long s.n., Megaceros flagellaris (Mitt.) Steph. AUSTRALIA, Cargill 885 (CANB), Dendroceros crispatus (Hook) Nees, AUSTRALIA, D.C. Cargill 28 (CANB), Pellia epiphylla (L.) Corda, ILLINOIS, Welsh s.n.
Fluorescence light microscopy and fluorochemical staining
Live specimens were collected from private land adjacent to the Shawnee National Forest in Jackson County, Illinois (37.627721, −89.260070). In order to detect callose in spores, excised sporophytes (n = 18) and 1 μm thick sections were collected on slides, covered by 1% aniline blue in 0.067 M Na2HPO4 (pH 8.5), placed in the dark at 4° C for 3-5 days, and rinsed in buffer. To visualize cellulose, resin-embedded thick-sections (1 μm) were placed on glass slides with a drop of Calcofluor white (Sigma-Aldrich) stain and a drop of 10% KOH buffer for 3 min. Controls were made using the respective buffers without aniline blue or Calcofluor white. All stained material was viewed with a Leica DM500B compound microscope (excitation filter equipped with ultraviolet fluorescence between 360-400nm). Images were collected digitally using a Q-Imaging Retiga 2000R digital camera. For images of Arabidopsis, 0.5 micrometer sections were taken from existing tissue blocks from a previous study (Paxson-Sowders et al. 2001). The sections were heat fixed to slides, the resin was removed with sodium methoxide (Sutherland and McCulley 1976), and then sections were mounted in 0.05% aniline blue in phosphate buffer (Smith and McCully 1978). Material was viewed with a Nikon Eclipse 80i compound microscope (excitation filter UV-2E/C DAPI, Excitation 325-375nm, Dichroic 400 LP, Emitter 435-485nm). Epifluorescent and DIC images of the same section for each stage were collected digitally using a Q-Imaging Retiga 2000R camera.
Transmission electron microscopy
Sporophytes of hornworts were dissected, fixed in 2% v/v glutaraldehyde in 0.05 M Na2HPO4 (pH 7.2), washed in buffer, post-fixed in 1% (w/v) OsO4 (15 min), rinsed in distilled water, and dehydrated in a graded ethanol series ending with 3 changes of 100% ethanol. Fixed tissue infiltrated in Spurr’s resin (Electron Microscopy Sciences, Hatfield, Pennsylvania, USA), was embedded in Beem® capsules and thermal cured in a 65° C oven. Tissue in London Resin White (LR White) (London Resin Company, Berkshire, UK) was infiltrated slowly over 4d by increasing percentage of resin to ethanol. Plant material was placed in molds with fresh resin and cured for 2d at 65°C. Semithin sections (250–750 nm) were mounted on glass slides and stained with 1.5% toluidine blue in distilled water to monitor for stage of development using light microscopy. Thin sections (90 nm) were collected onto 200 mesh Ni grids and post-stained with uranyl acetate (UA) for 3 min and Reynold’s lead citrate (Pb) for 30 sec. Samples were viewed and micrographs were digitally collected in an Hitachi H7650.
Immunogold labeling
The immunogold labeling procedure follows that in Lopez et al. (2017), and is briefly described herein: The primary monoclonal antibodies (MAbs) used were anti-1,3-β-glucan (callose), LM19 (unesterified HG), JIM7 (esterified HG), LM15 (xyloglucan), LM21 (mannans), and LM25 (galactoxyloglucans) (Table 1). Control grids were prepared by excluding the primary antibodies. Grids were blocked with 2.0 % bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, Missouri, USA) in 0.05M phosphate buffered saline (PBS) overnight at 4°C before incubating overnight on drops of a 1:20 dilution of the specified MAbs dissolved in 2% BSA/PBS. All grids were rinsed in 2% BSA/PBS 4x for 4 min each then placed on drops of a 1:20 dilution of goat-anti-rat or goat-anti-mouse IgG-gold (Sigma-Aldrich) secondary antibody for 30 min followed by 4 rinses in PBS (4 min each) and then rinsed 4x in dH2O. Sections were post-stained in 2% aqueous UA for 3 min and Reynold’s Pb citrate for 30 s then allowed to air dry at room temperature. Sections were examined and digital images were captured in an Hitachi H7650 TEM.
Table 1.
Monoclonal primary antibodies used in this study, their epitope specificities and references.
| Antibody | Antigen (s)/ Epitope | Reference/ Source |
|---|---|---|
| Anticallose | Callose/ (1,3)-β-linked penta-to-hexa-glucan | Meikle et al., 1991/ Biosupplies Australia |
| JIM7 | Partially methyl-esterified epitopes of homogalacturonan | Knox et al., 1990; Verhertbruggen et al., 2009/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM19 | Homogalacturonan/ Un-esterified | Verhertbruggen et al., 2009a/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM20 | Homogalacturonan/ Methyl-esterified | Verhertbruggen et al., 2009a/ J. P. Knox PlantProbes, University of Leeds, UK |
| LM25 | Galactoxylated xyloglucans | Pedersen et al., 2012/ J. P. Knox PlantProbes, University of Leeds, UK |
Results
Following meiosis, nascent spores of Phaeoceros and Notothylas each contain large starch-filled plastids and are surrounded by a thick spore mother cell wall that is devoid of callose (Fig. 1a). Callose first appears early in spore wall development as a discontinuous layer (Fig. 1a), that eventually surrounds the developing spore (Fig. 1b). As the tetrahedral spores mature, the cytoplasm increases in volume and the spores fill most of the space within the spore mother cell wall (Fig. 1c). The spore wall at this stage consists of an amorphous thin outer sporopollenin-filled layer (exine 1) that undulates to form the sculptured sporoderm (Fig. 1c), and an extensive inner exine (exine 2) that contains scattered aggregates of sporopollenin (Figs. 1d, e). As evidenced by callose epitope labels inside of exine 2 and outside of the plasmalemma, callose is a component of the intine from its inception (Fig. 1d). Anticallose epitopes are also labeled in the aperture located along the trilete mark on the proximal spore surface (Figs. 1b, e), and the equatorial girdle that encircles the spore (Fig. 1b). Both of these regions contain little sporopollenin throughout development and consequently they detach easily from the remainder of the spore wall, thereby facilitating germination.
Figure 1. Initiation of the callosic intine.
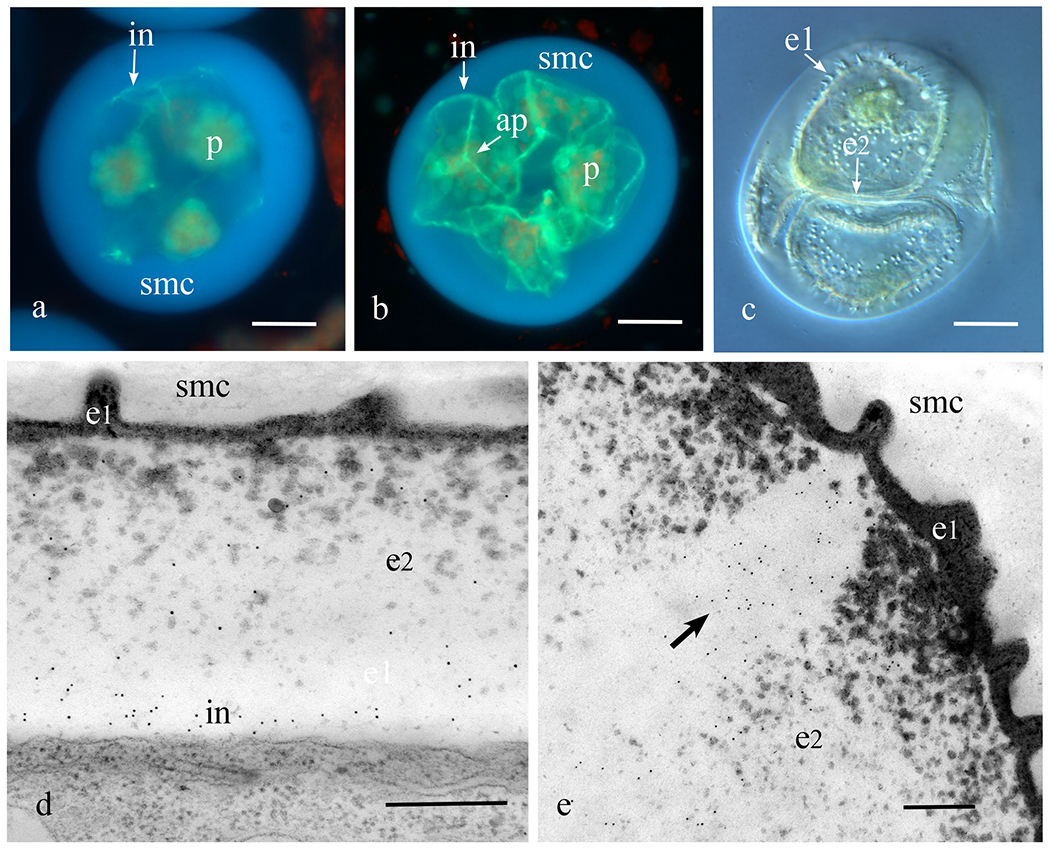
a-c. Phaeoceros carolinianus. a. Aniline blue fluorescence of a young tetrad enclosed in the spherical spore mother cell (smc) wall showing fluorescence of the callosic intine (in) that develops in patches around the spores; each spore contains a single starch-laden plastid (p). b. Developing spores with single plastids (p) still enclosed in spore mother cell (smc) wall showing newly produced callosic intines (in). The trilete aperture (ap) on the proximal spore surface is enriched in callose. c. DIC image of developing spores enclosed in the mother cell wall showing ornamentation of the outer exine (e1) and inner exine (e2) not yet filled with sporopollenin. Intine is expanding at this stage. d, e. Notothylas orbicularis TEM immunogold labeling with anticallose antibody. e. Developing intine with abundant labels. Outer exine (e1) undulates to form the ornamentation and the inner exine (e2) is filling with sporopollenin. e. Aperture along trilete mark contains callose (arrow points to gold labels) in developing spore wall. Bars: a-c = 20μm, d, e = 2μm.
The intine of Phaeoceros and Notothylas contains callose throughout maturation (Figs. 2a–f). The band of callose that is localized in the intine of spores in tetrads (Figs. 2a, b) and separated mature spores (Figs. 2c, d) is readily visible with aniline blue fluorescence. The spore wall in the mature spore of all genera examined consists of an exine that is impregnated with sporopollenin and a thinner continuous intine (Figs. 2e–j). Anticallose immunolabeling in Leiosporoceros, Anthoceros and Megaceros reveals similar patterns of epitope labeling restricted to the band of intine (Figs. 2g–i).
Figure 2. Mature to nearly mature spores with fully developed intine.
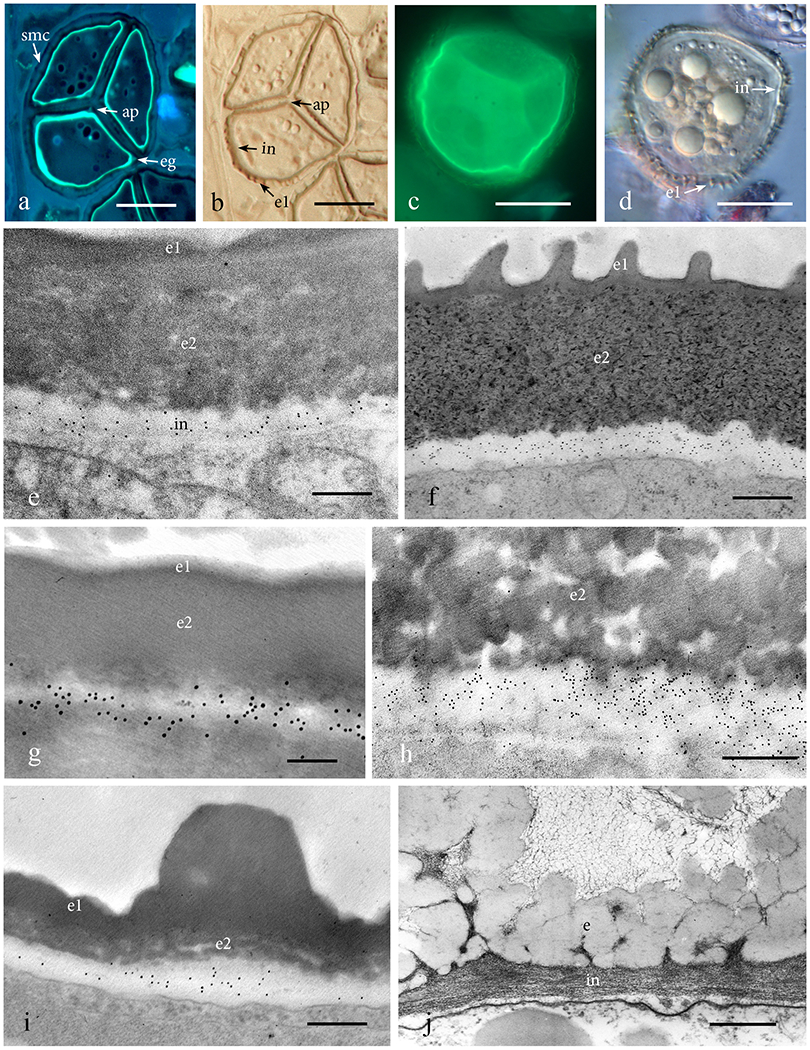
a, b. Serial sections of a nearly mature Phaeoceros carolinianus spores in a tetrad surrounded by spore mother cell (smc) wall. a. Aniline blue fluorescence reveals callose in the intine, aperture (ap) and equatorial girdle (eg). b. DIC serial section of 2a shows aperture (ap) and exine outside of the intine with exine 1 (e1) forming the surface ornamentation of the spore. c, d. Phaeoceros carolinianus mature spore not enclosed in spore mother cell wall. c. Aniline blue fluorescence of intine. d. Intine (in) interior to the exine with outer layer (e1) forming ornamentation. e-i. Immunogold labeling with anticallose antibody in diverse hornwort genera. Only the intine (in) is labeled for callose. No labelling occurs in the outer exine (e1) and inner exine (e2). e. Phaeoceros carolinianus. f. Notothylas orbicularis. g. Leiosporoceros dussii. h. Anthoceros agrestis. i. Megaceros flagellaris. j. The spore wall of Dendroceros crispatus consists of two layers, exine (e) and fibrous intine (in), both of which lack labeling with the anticallose MAb. Bars: a, b = 20μm; c, d = 15μm; g = 100nm; h, j = 500nm; e, i = 250nm.
Dendroceros is the only hornwort of the six genera probed that lacks callose in the intine (Fig. 2j). It is also the only taxon that produces multicellular endosporic spores. The exine in this genus is folded, enabling the spore to expand as it undergoes mitosis while remaining surrounded by the spore wall (Figs. 2j, 3a). The anticallose MAb localizes callose only in developing cell walls in Dendroceros multicellular spores (Fig. 3b). A similar occurrence of callose is evident in multicellular spores of the liverwort Pellia when treated with aniline blue (Fig. 3c). Young cell walls of this liverwort fluoresce as cell plates are laid down. Probing of the Arabidopsis pollen grain reveals the same pattern. Although callose is prominent in the spore mother cell wall around the young tetrad of microspores (Fig. 3d), this polysaccharide does not appear in the pollen grain until mitosis ensues (Figs. 3e, f). The wall of the microspore contains no callose, while two-celled pollen grains have callose in both the intine and between the generative and vegetative cells (vegetative cell wall) (Figs. 3e, f).
Figure 3. Callose in multicellular spores of plants.
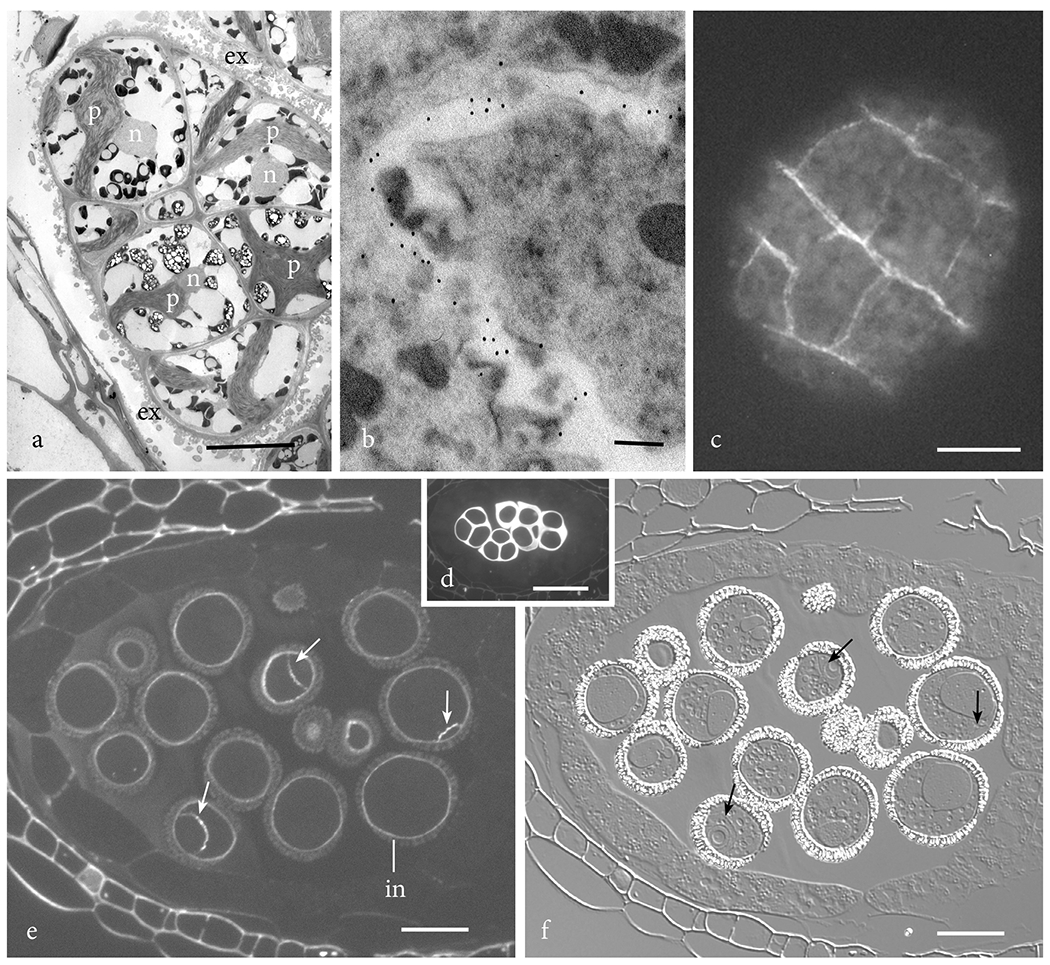
a. Dendroceros granulatus multicellular spore showing spore wall ornamentation contributed by the exine (ex) and cells each with a nucleus (n) and star-shaped plastid (p). b. Anticallose epitope labels in Dendroceors granulatus are localized in young cell walls in the multicellular spore. c. The liverwort Pellia epiphylla produces multicellular spores that contain callose in newly produced cell walls as seen with aniline blue fluorescence. d-f. Arabidopsis thalianus sections of anthers. d. Aniline blue fluorescence of young tetrads of microspores surrounded by a thick callosic wall prior to spore wall development. e. Aniline blue fluorescence of two-celled pollen showing presence of callose in the intine (in) and generative cell wall (arrows). f. DIC image of same section as e. showing pollen wall outside of intine and location of callose-containing generative cell wall (arrows) in two-celled pollen grains. Bars: a, c = 10μm; b = 100nm; d = 25 μm; e, f = 12.5 μm.
In addition to callose, the intine of hornwort spores contains cellulose, pectins and hemicellulose (Fig. 4). Calcofluor fluorescence reveals the presence of cellulose in the spore intine, and in the exine region of the aperture and equatorial girdle that is devoid of sporopollenin (Fig. 4a). Immunogold labeling localizes abundant epitopes for esterified (JIM7 and LM20) (Figs. 4b, c) and unesterified (LM19) (Fig. 4d) homogalacturonan pectins. The presence of hemicellulose is verified through localization of LM25 epitopes (Fig. 4e).
Figure 4. Polysaccharides constituents in the intine of hornworts.
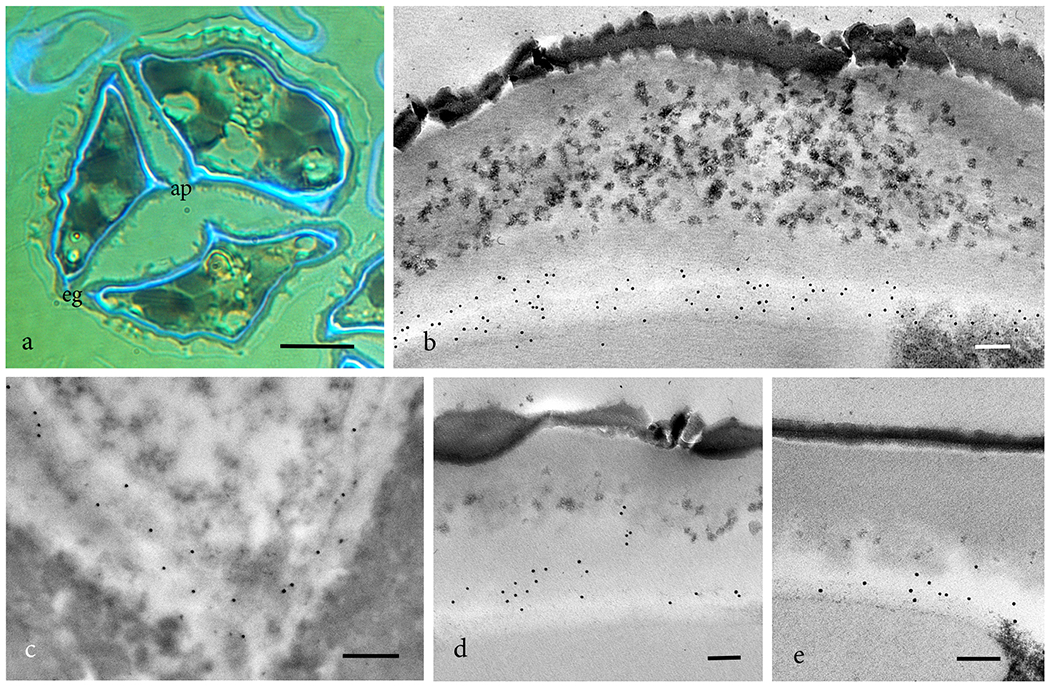
a. Phaeoceros carolinianus. Calcofluor fluorescence in a sectioned nearly mature tetrad identifies cellulose as a component of the intine, aperture (ap) and equatorial girdle (eg). b-e. TEM immunogold labeling with monoclonal antibodies. b. Leiosporoceros dussii JIM7 labeling for esterified homogalacturonans pectin. c. Phaeoceros carolinianus LM20 labeling for esterified homogalacturonans pectin. d. Leiosporoceros dussii LM19 labeling for unesterified homogalacturonans pectin. e. Leiosporoceros dussii LM25 labeling for galactoxyloglucan hemicellulose. Bars: a = 10μm; b-e = 100nm.
Discussion
As a polymer that is rapidly synthesized and transient in nature, callose plays important roles in building and maintaining wall integrity for growth and response to biotic and abiotic stress (Ellinger and Voigt 2014). Its involvement in isolation and protection has long been known, but the precise role in processes such as sporogenesis is less clear. Callose may be deposited before and/or after meiosis during sporogenesis, and it typically disappears as the spore wall is laid down. In contrast to other land plants, callose in hornworts is a constituent of the inner spore wall from its inception until maturity. This location for callose is unique among land plants and widespread across hornwort diversity, as it occurs in four of five hornwort families we examined. It is logical to surmise that as a spore wall constituent, callose in hornworts may have multiple functions. First, because callose is hydrophilic, it is possible it is involved in water retention in the intine while providing a protective matrix through which certain solutes may be transported (Heslop-Harrison 1964; Yim and Bradford 1998; Barskaya and Balin 1971; Vithanage et al. 1980; Johri 2012). Secondly, the presence of hydrophilic callose in the intine may reduce water loss when spores are dry and provide the cell wall with mechanical flexibility to withstand desiccation (Vithanage et al. 1980; Herburger and Holzinger 2015; Dong et al. 2005). And finally, the presence of callose in the aperture and equatorial girdle may provide enhanced strength, while taking up and retaining water during germination (Parre and Geitmann 2005). The remainder of the exine is densely packed with sporopollenin and forms an immutable barrier that ensures the spore boundary is firm until water is available and during imbibition.
The absence of callose in Dendroceros is most likely related to the unique spore development in this epiphytic plant. Characteristic of many epiphytic bryophytes, Dendroceros spores are precocious and endosporic (Schuette and Renzaglia 2010). Visibly different, the green spores of Dendroceros have thin walls that readily expand, allowing the spores to increase in size as they become multicellular, filling the space in the spore chamber (Renzaglia 1978). The undulating exine is relatively thin compared with other hornworts and stretches, leveling the folds, as the spore increases in size. As a result, the fibrous intine effectively delineates the spore boundary and provides scaffolding for support and solute movement. Clearly, in this derived genus, spore wall composition and architecture are modified and adapted to the epiphytic habitat.
Among the remaining bryophytes, callose is prominent during sporogenesis in liverworts and is associated with sculptoderm formation, performing roles in determining spore wall patterns in the spore mother cell (Brown and Lemmon 1987). In Sphaerocarpos, a thick layer of callose is deposited prior to meiosis and surrounds the entire spore mother cell (Dubois-Tylski 1981; Renzaglia et al. 2015). The precursors for spore wall ornamentation form within this callosic layer and serve as a template for the deposition of the outer exine, which is progressively built around white line lamellae and reinforced with sporopollenin. This arrangement of callose around the entire spore mother cell and in the intersporal septum are key to the development of permanent tetrads in this liverwort (Renzaglia et al. 2015). There is but a sole unequivocal report of callose in moss sporogenesis, and that is in the aperture of spores of Physcomitrella patens (Schuette et al. 2009). This elusive post-meiotic deposition of callose has been suggested to function in preparing spores for germination.
Across pteridophytes, callose has been reported in sporogenesis of heterosporous lycophytes and homosporous ferns. In Selaginella, pre-meiotic callose deposition surrounding the myriad of microsporocytes and sole megasporocyte per sporangium determines their viability (Horner and Beltz 1970). A systematic and thorough study across ferns and lycophytes to document the presence of callose during sporogenesis awaits to be undertaken and is warranted.
In angiosperms, callose is the primary carbohydrate in the wall surrounding developing microspores and pollen tubes (Drabkova and Honys 2017). Callose is produced by the meiocyte during meiosis, separates spores in the early tetrad as show here in Arabidopsis, and is involved in exine development. In contrast to liverworts, the exine does not form directly in the callose, but the callosic layer serves to restrict the polysaccharide primeexine within which the exine forms (Dong et al. 2005; Enns et al. 2005; Nishikawa et al. 2005). In Arabidopsis there are twelve callose synthases genes of which CalS 5, 9, 10, 11 and 12 are involved in microsporogenesis and male gametophyte development (Drabkova and Honys 2017; Lu et al. 2014). As evidenced by mutational studies, CalS5 is necessary for pollen development. In cals5 mutants, the loss of callose prevents deposition of primexine, which serves as a template for constructing the sporopollenin exine in the pollen grain (Verma and Hong 2001; Xie et al. 2009). Aberrant pollen exine patterning and subsequent pollen grain sterility are the result (Dong et al. 2005; Xie et al. 2009). Comparatively, CalS11 and CalS12 synthesize callose between microspores in the tetrad for subsequent separation. Double mutation of these redundant complexes resulted in serious reduction in callose production and degeneration of the microspores (Lu et al. 2014).
In addition to callose, polysaccharide constituents in the intine of hornworts include cellulose, pectin, and hemicellulose as do the plant primary cell walls (Heslop-Harrison 1968). A similar composition between intine and primary cell wall is logical given that upon germination, newly generated cell plates/walls are continuous with the intine (Bhandari 1984; Park 2001). Indeed, in most ferns, the intine is not present at the time of spore dispersal, rather, it develops during spore germination (Lugardon 1990; Heslop-Harrison 1979; Horner and Beltz 1970), reinforcing this continuity and similarity between intine and new cell walls. Only homosporous fern species such as Hymenophyllum, Sphaerocionium and Grammitis exhibiting precocious germination syndromes develop an intine prior to spore release (Tyron and Lugardon 2012; Uehara and Kurita 1989). As demonstrated in the present study, new cell walls in the precocious and endosporic bryophytes, Dendroceros and Pellia, similarly contain callose, while intines in the single-celled spores do not. A different callose synthase gene, CalS9, is required for the entry of microspores into the first and second mitotic divisions (Drabkova and Honys 2017). Consequently, it seems plausible that CalS9 may be responsible for the hornwort callose in the intine.
Seed plant intines are present in microspores and are continuous with primary walls that are produced and surround cells within the pollen grain or developing male gametophytes. A cellulose/pectin scaffolding characterizes these cell walls and contributes to the structural integrity of pollen grains as demonstrated by knock-out mutational studies (Persson et al. 2007). Although microspores lack callose in their walls, this polymer appears when mitosis ensues, as shown here in Arabidopsis. One day after pollen germination in Gnetum and Ginkgo, callose is deposited in the cellulosic walls of nascent male gametophytes. Eight days after germination, the sporoderm is shed, leaving a callose-coated gametophyte. In some Pinus species, callose is present prior to the emergence of the pollen tube in association with the aperture region and the prothallial cells. After germination, a consistent layer of callose wall can be seen in the growing pollen tube in all seed plants (Abercrombie et al. 2011).
In addition to the genetic diversity inherent in their meiotic origin, spores serve important roles in dispersal and colonization of new environments. The production of the sporopollenin-coated spore wall was key to the radiation and persistence of plants on land. Callose is extensively utilized in developing spores, especially in the production of spore walls, in many embryophytes. By corroborating data from fluorescence and transmission electron microscopy, we have demonstrated that callose is present and persistent in spore walls of the hornworts Phaeoceros, Anthoceros, Leiosporoceros, Notothylas and Megaceros, but not Dendroceros. While the localization and temporal deposition are different across land plants, the presence of callose in hornworts provides evidence that callose is an integral polysaccharide involved in sporogenesis of the vast majority of embryophytes.
Acknowledgements:
This work was supported by grants from the National Science Foundation (NSF 1758497) and the National Institutes of Health (NIH 107760).
Literature Cited
- Abercrombie JM, O’Meara BC, Moffatt AR, Williams JH (2011) Developmental evolution of flowering plant pollen tube cell walls: callose synthase (CalS) gene expression patterns. EvoDevo 2: 14. doi. 10.1186/2041-9139-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barskaya EI, Balina NV (1971) The role of callose in plant anthers. Fiziol Rast 18: 716–721. [Google Scholar]
- Bhandari N (1984) The microsporangium. In: Johri BM (ed) Embryology of angiosperms, Springer, Berlin, Heidelburg, pp 53–121. doi. 10.1007/978-3-642-69302-1_2 [DOI] [Google Scholar]
- Brown RC, Lemmon BE (1987) Involvement of callose in determination of exine patterning in three hepatics of the subclass Jungermanniidae. Mem NY Botan G 45:111–121. [Google Scholar]
- Brown RC, Lemmon BE (1990) Sporogenesis in bryophytes. In: Blackmore SB, Knox RB (eds) Microspores: evolution and ontogeny Academic Press, London, pp 55–94. [Google Scholar]
- Brown RC, Lemmon BE (2011) Spores before sporophytes: hypothesizing the origin of sporogenesis at the algal–plant transition. New Phytol 190: 875–881. doi: 10.1111/j.1469-8137.2011.03709.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Shimamura M, Villarreal JC, Renzaglia KS (2015) Spores of relictual bryophytes: Diverse adaptations to life on land. Review of Palaeobot Palyno 216: 1–17. doi. 10.1016/j.revpalbo.2015.01.004 [DOI] [Google Scholar]
- Dong X, Hong Z, Sivaramakrshnan M, Mahfouz M, Verma DP (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42: 315–328. doi: 10.1007/s00425-008-0812-3 [DOI] [PubMed] [Google Scholar]
- Drabkova LZ, Honys D (2017) Evolutionary history of callose synthases in terrestrial plants with emphasis on proteins involved in male gametophyte development. PloS one, 12(11) e0187331. doi. 10.1371/journal.pone.0187331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Tylski T (1981) Utilisation de fluorochromes pour l’observation des parois cellulaires chez trois especes de Closterium (Desmidiales) au cours de leur reproduction sexuee. Cryptogam Algol 1: 277–287. [Google Scholar]
- Ellinger D, Voigt CA (2014) Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot 114: 1349–1358. doi. 10.1093/aob/mcu120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE (2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol 58: 333–349. doi. 10.1007/s11103-005-4526-7 [DOI] [PubMed] [Google Scholar]
- Herburger K, Holzinger A (2015) Localization and quantification of callose in the streptophyte green algae Zygnema and Klebsormidium: correlation with desiccation tolerance. Plant Cell Physiol 56: 2259–2270. doi. 10.1093/pcp/pcv139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J (1964) Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In: Linskens HF (ed) Pollen Physiology and Fertilization. NorthHolland, Amsterdam, pp 29–47. [Google Scholar]
- Heslop-Harrison J (1968) Pollen wall development. Science 161: 230–237. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J (1979) An interpretation of the hydrodynamics of pollen. Am J Bot: 737–743. doi. 10.1002/j.1537-2197.1979.tb06277.x [DOI] [Google Scholar]
- Horner HT Jr, Beltz CK (1970) Cellular differentiation of heterospory in Selaginella. Protoplasma 71: 335–341. doi. 10.1007/BF01279640 [DOI] [Google Scholar]
- Johri BM (2012) Embryology of angiosperms. Springer Science & Business Media. [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS (2012) Major transitions in the evolution of early land plants: a bryological perspective. Ann of Bot 109: 851–871. doi. 10.1093/aob/mcs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RA, Mansouri K, Henry JS, Flowers ND, Vaughn KC, Renzaglia KS (2017) Immunogold localization of molecular constituents associated with basal bodies, flagella, and extracellular matrices in male gametes of land plants. Bio Protoc 7, Iss 21, e2599. doi: 10.21769/BioProtoc.2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Chai M, Yang J, Ning G, Wang G, Ma H (2014) The Arabidopsis CALLOSE DEFECTIVE MICROSPORE1 gene is required for male fertility through regulating callose metabolism during microsporogenesis. Plant Physiol 164: 1893–1904. doi. 10.1104/pp.113.233387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugardon B (1990) Pteridophyte sporogenesis: a survey of spore wall ontogeny and fine structure in a polyphyletic plant group. In: Blackmore S, Knox RB (eds) Microspores: Evolution and Ontogeny, Academic Press, pp 95–120. [Google Scholar]
- Neidhart H (1979) Comparative studies of sporogenesis in bryophytes. In: Clarke GCS, Duckett JG (eds.) Bryophyte Systematics, Academic Press, London and New York, pp 251–280. [Google Scholar]
- Nishikawa SI, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (β-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5: 22. doi. 10.1186/1471-2229-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parre E, Geitmann A (2005) More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol 137: 274–286. doi/ 10.1104/pp.104.050773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK (2001) Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol 126: 899–909. doi. 10.1104/pp.126.2.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation durig pollen development in Arabidopsis. Plant Physio 127: 1739–1749. doi. 10.1104/pp.010517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR (2007) Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci 104: 15566–15571. doi/ 10.1073/pnas.0706592104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piršelová B, Matušíková I (2013) Callose: the plant cell wall polysaccharide with multiple biological functions. Acta Physiol Plant 35: 635–644. doi. 10.1007/s11738-012-1103-y [DOI] [Google Scholar]
- Radja A, Horsley EM, Lavrentovich MO, Sweeney AM (2019) Pollen Cell Wall Patterns Form from Modulated Phases. Cell 176: 856–68. doi. 10.1016/j.cell.2019.01.014 [DOI] [PubMed] [Google Scholar]
- Renzaglia KS (1978) Comparative morphology and developmental anatomy of the Anthocerotophyta. J Hattori Bot Lab 44: 31–90. [Google Scholar]
- Renzaglia KS, Duff RJ, Nickrent DL, Garbary DJ (2000) Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philos T Roy Soc B 355: 769–793. doi. 10.1098/rstb.2000.0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Schuette S, Duff RJ, Ligrone R, Shaw AJ, Mishler BD, Duckett JG (2007) Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist 110: 179–213. doi.org/10.1639/0007-2745(2007)110[179:BPATMA]2.0.CO;2 [Google Scholar]
- Renzaglia KS, Lopez RA, Johnson EE (2015) Callose is integral to the development of permanent tetrads in the liverwort Sphaerocarpos. Planta 241: 615–627. doi. 10.1007/s00425-014-2199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway JE (1965) Some aspects of morphogenesis, biotic coaction, and ultrastructure of the genus Anthoceros. PhD Thesis, University of Texas, Texas. [Google Scholar]
- Schuette S, Wood AJ, Geisler M, Geisler-Lee J, Ligrone R, Renzaglia KS (2009) Novel localization of callose in the spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Ann Bot 103: 749–756. doi. 10.1093/aob/mcn268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuette S, Renzaglia KS (2010) Development of multicellular spores in the hornwort genus Dendroceros (Dendrocerotaceae, Anthocerotophyta) and the occurrence of endospory in Bryophytes. Nova Hedwigia 91: 301–316. doi. 10.1127/0029-5035/2010/0091-0301 [DOI] [Google Scholar]
- Smith MM, McCully ME (1978) A critical evaluation of the specificity of aniline blue induced fluorescence. Protoplasma 95: 229–254. doi. 10.1007/BF01294453 [DOI] [Google Scholar]
- Sutherland J, McCully ME (1976) A note on the structural changes in the walls of pericycle cells initiating lateral root meristems in Zea mays. Can J Bot 54: 2083–2087. doi. 10.1139/b76-222 [DOI] [Google Scholar]
- Tryon AF, Lugardon B (2012) Spores of the Pteridophyta: surface, wall structure, and diversity based on electron microscope studies, Springer Science & Business Media. [Google Scholar]
- Uehara K, Kurita S (1989) An ultrastructural study of spore wall morphogenesis in Equisetum arvense. Am J Bot 76: 939–951. doi. 10.1002/j.1537-2197.1989.tb15074.x [DOI] [Google Scholar]
- Verma DPS, Hong Z (2001) Plant callose synthase complexes. Plant Mol Biol 47: 693–701. doi. 10.1023/A:1013679111111 [DOI] [PubMed] [Google Scholar]
- Vithanage HIMV, Gleeson PA, Clarke AE (1980) The nature of callose produced during self-pollination in Secale cereale. Planta 148: 498–509. doi. 10.1007/BF00552666 [DOI] [PubMed] [Google Scholar]
- Wallace S, Fleming A, Wellman CH, Beerling DJ (2011) Evolutionary development of the plant spore and pollen wall. AoB PLANTS, plr027. doi. 10.1093/aobpla/plr027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Wang X, Hong Z (2009) Precocious pollen germination in Arabidopsis plants with altered callose deposition during microsporogenesis. Planta 231: 809–823. doi: 10.1007/s00425-009-1091-3 [DOI] [PubMed] [Google Scholar]
- Yim K, Bradford K (1998) Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskmelon seeds. doi. 10.1104/pp.118.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]


