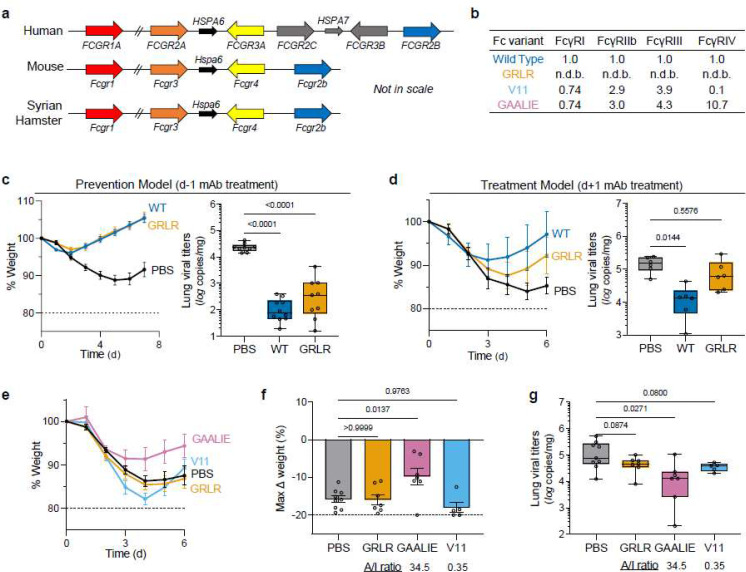
Fig. 1: Contribution of Fc effector function to the protective activity of neutralizing anti-SARS-CoV-2 mAbs in hamster infection models.
a, Overview of the FcγR locus organization in humans, mice, and Syrian hamsters. b, Fc variants of human IgG1 were evaluated for their affinity for hamster FcγRs. Numbers indicate the fold-change in affinity compared to wild-type human IgG1. n.d.b., no detectable binding. c, d, Wild-type and FcR null (GRLR) variants of REGN mAb cocktail (c) or S309 mAb (d) were administered i.v. (5 mg/kg) to Syrian hamsters one day before (prevention model, c) or after (therapy model, d) i.n. challenge with SARS-CoV-2 (NYC isolate, 105 pfu) (n=9–10 hamsters per group from two independent experiments for c and n=6 hamsters per group from two independent experiments for d). Hamsters were monitored for weight loss (left; mean ± s.e.m.) and lung viral titers (right, analyzed on day 7 (c) or 6 (d) post-infection) were compared between treatment groups by one-way ANOVA (Bonferroni post hoc analysis adjusted for multiple comparisons). P values are indicated. e-g, SARS-CoV-2-infected hamsters (105 pfu, NYC isolate) were treated on day 1 post-infection with Fc variants of the REGN mAb cocktail (5 mg/kg, i.v.) exhibit differential hamster FcγR binding affinity and A/I ratio (calculated based on FcγRIV/FcγRIIb affinity). Weight loss (e, plotted over time (mean ± s.e.m.) or f, as max change) and lung viral titers (g, assessed on day 6 post-infection) were compared by one-way ANOVA (Bonferroni post hoc analysis adjusted for multiple comparisons). P values are indicated. n=5–9 hamsters per group from two independent experiments.