Figure 4. Schematics of the TIM3/Gal9 pathway in innate immune cells.
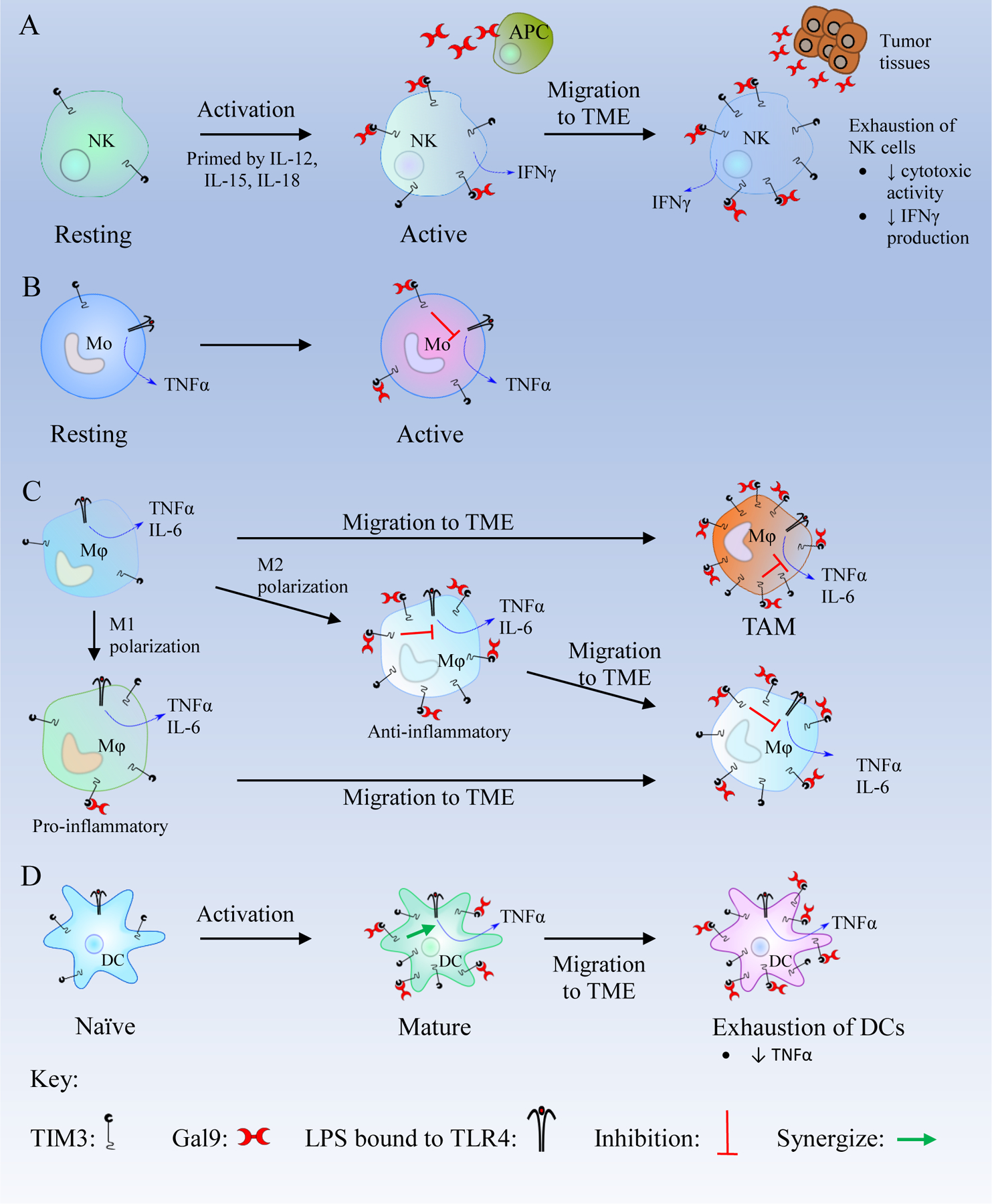
(A) Resting natural killer (NK) cells become active in the presence of IL-12, IL-15, and IL-18 and express more T cell immunoglobulin and mucin-containing protein 3 (TIM3). In active NK cells, TIM3/Galectin 9 (Gal9) binding enhances interferon γ (IFNγ) secretion. After migration to the tumor microenvironment (TME), NK cells are exposed to high levels of Gal9 which leads to their exhaustion.
(B) Active monocytes (Mo) express a high number of TIM3 receptors. The TIM3/Gal9 interaction inhibits the Lipopolysaccharide (LPS)/Toll like receptor 4 (TLR4) pathway and decreases the secretion of TNFα. (C) Macrophages (Mφ) in the TME overexpress TIM3. Macrophages are polarized to M1 and M2 phenotypes. M1 macrophages are pro-inflammatory and express a low level of TIM3. M1 macrophages can migrate to the TME and may transform to M2 macrophages. M2 macrophages are anti-inflammatory and express high levels of TIM3. TIM3/Gal9 binding in M2 macrophages inhibits LPS/TLR4 pathway and decreases the production of inflammatory cytokines like Tumor Necrosis Factor α (TNFα), interleukin-6 (IL-6), and IL-12. After migrating to the TME, M2 macrophages overexpress TIM3, and the TIM3/Gal9 binding leads to the inhibition of the LPS/TLR4 pathway to suppress the immune response. (D) Mature DCs express a high level of TIM3, and the TIM3/Gal9 binding synergizes with the LPS/TLR4 pathway which produces TNFα. In Dendritic cells (DCs) within the TME, the TIM3/Gal9 interaction suppresses CXCL9 expression, thus inhibiting innate immunity and promote tumor growth.
